| CPC H01M 4/525 (2013.01) [C01G 53/44 (2013.01); H01M 4/0471 (2013.01); H01M 4/0497 (2013.01); H01M 4/505 (2013.01); H01M 10/0525 (2013.01); C01P 2004/61 (2013.01); C01P 2006/40 (2013.01); H01M 2004/021 (2013.01); H01M 2004/028 (2013.01)] | 13 Claims |
|
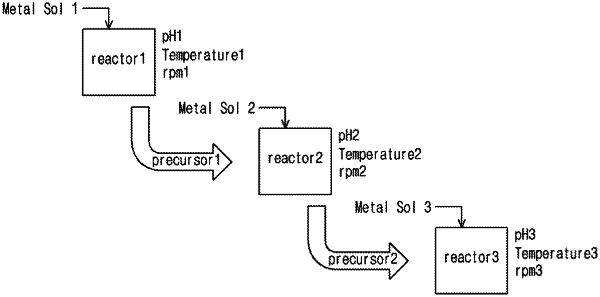
1. A method of preparing a positive electrode active material precursor for a lithium secondary battery, comprising:
a first step of forming a reaction solution by adding a transition metal-containing solution containing at least one selected from nickel, cobalt, and manganese, an ammonium ion-containing solution, and a basic aqueous solution to a first reactor and performing a co-precipitation reaction under a first pH condition to form transition metal hydroxide seeds;
a second step of performing a co-precipitation reaction under a second pH condition while transferring the reaction solution of the first reactor to a second reactor to grow the transition metal hydroxide seeds;
a third step of performing a co-precipitation reaction under a third pH condition while transferring a reaction solution of the second reactor to a third reactor to grow transition metal hydroxide particles; and
a fourth step of recovering the transition metal hydroxide particles from the third reactor,
wherein reaction conditions of the first reactor, the second reactor, and the third reactor satisfy Equation 1 and Equation 2
Equation 1: first pH>second pH≥third pH
Equation 2: 4≤temperature of the reaction solution (° C.)/pH of the reaction solution ≤6.
|