| CPC H01M 10/0562 (2013.01) [C01B 25/14 (2013.01); H01M 10/0525 (2013.01); H01M 10/0585 (2013.01); C01P 2002/30 (2013.01); C01P 2002/72 (2013.01); C01P 2002/74 (2013.01); C01P 2002/77 (2013.01); C01P 2002/82 (2013.01); C01P 2002/86 (2013.01); C01P 2006/40 (2013.01); H01M 2300/008 (2013.01)] | 9 Claims |
|
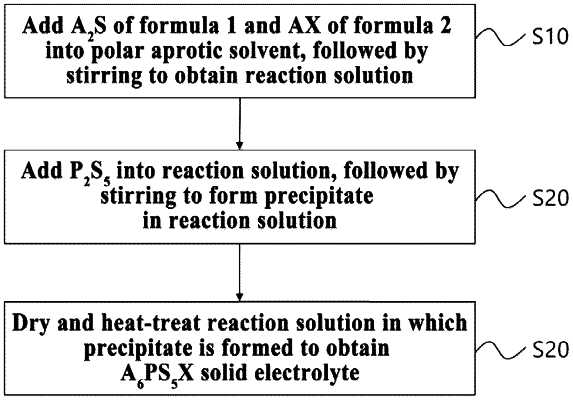
1. A method of manufacturing an argyrodite-type solid electrolyte, comprising:
a first step of adding precursors represented by the following Formulas 1 and 2 into a polar aprotic solvent, followed by stirring to obtain a reaction solution;
a second step of adding P2S5 into the stirred reaction solution, followed by further stirring to form a precipitate obtained as a result of a reaction in the reaction solution; and
a third step of drying and heat-treating the reaction solution in which the precipitate is formed to obtain a solid electrolyte:
wherein, in the second step,
after solvated P2S62− is formed in the reaction solution, the P2S62− is converted into solvated PS43−, and a portion of the PS43− forms amorphous A6PS5X as a precipitate along with A+, A2S, and AX, and
the reaction solution comprises a deposit including the precipitate and a supernatant above the deposit, and the reaction is performed until a fraction of PS43− ions becomes higher than a fraction of P2S62− ions in the supernatant, and a fraction of the precipitated A6PS5X becomes higher than a fraction of A2S in the deposit,
A2S [Formula 1]
AX [Formula 2]
wherein A represents an alkali metal, and X represents an element of the halogen group.
|