| CPC G16H 10/20 (2018.01) [G06N 20/00 (2019.01)] | 20 Claims |
|
1. A method, comprising:
obtaining, by a device, historical clinical trial information and historical rate of recruitment (RoR) information,
wherein the historical clinical trial information includes historical information concerning incidence and/or prevalence of a disease;
preprocessing, by the device, the historical clinical trial information and the historical RoR information,
wherein preprocessing the historical clinical trial information and the historical RoR information comprises removing confidential data in the historical clinical trial information and the historical RoR information;
training, by the device, a plurality of machine learning models based on the historical clinical trial information and the historical RoR information;
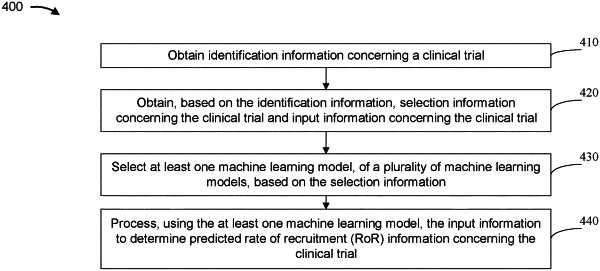
obtaining, by the device, identification information concerning a clinical trial,
wherein the identification information includes information associated with a protocol that concerns the clinical trial;
obtaining, by the device and based on the identification information, selection information concerning the clinical trial and input information concerning the clinical trial;
selecting, by the device, at least one machine learning model, of the plurality of machine learning models, based on using at least one additional machine learning model and the selection information,
wherein the at least one additional machine learning model is trained based on historical identification information and historical selection information to determine an association between the selection information and the at least one machine learning model, and
wherein selecting the at least one machine learning model comprises:
identifying the plurality of machine learning models,
determining one or more elements based on the selection information and the identification information,
processing the one or more elements,
wherein processing the one or more elements comprises identifying a particular element, of the one or more elements, associated with a relationship of high predictive significance with the at least one machine learning model, and
selecting the at least one machine learning model based on the particular element;
processing, by the device and using the at least one machine learning model, the input information, the historical clinical trial information, the historical information concerning incidence and/or prevalence of the disease, and the information associated with the protocol that concerns the clinical trial;
determining, by the device and based on processing the input information, the historical clinical trial information, the historical information concerning incidence and/or prevalence of the disease, and the information associated with the protocol that concerns the clinical trial, predicted RoR information concerning the clinical trial;
obtaining, by the device, present RoR information concerning the clinical trial;
determining, by the device, a status of the clinical trial based on the present RoR information and the predicted RoR information;
selectively:
displaying, by the device, a recommendation that a duration of the clinical trial be extended and/or a number of sites be increased when the status indicates that the clinical trial is underperforming, and
displaying, by the device, a recommendation that the duration of the clinical trial be reduced and/or the number of sites be decreased when the status indicates that the clinical trial is overperforming;
updating, by the device, the at least one machine learning model based on determining the status; and
processing, by the device and using the at least one machine learning model after the at least one machine learning model has been updated, the present RoR information, the predicted RoR information, the status, and the input information.
|