| CPC C12N 5/0634 (2013.01) [A61K 35/15 (2013.01); A61K 35/44 (2013.01); C12N 2500/02 (2013.01); C12N 2501/115 (2013.01); C12N 2501/125 (2013.01); C12N 2501/155 (2013.01); C12N 2501/165 (2013.01); C12N 2501/2306 (2013.01); C12N 2501/2311 (2013.01); C12N 2501/26 (2013.01); C12N 2501/727 (2013.01); C12N 2502/11 (2013.01); C12N 2506/45 (2013.01); C12N 2510/00 (2013.01); C12N 2533/50 (2013.01)] | 12 Claims |
|
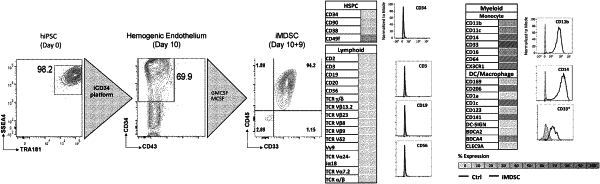
1. An in vitro method of generating a population of induced immune regulatory cells, comprising:
(i) obtaining induced definitive hemogenic endothelium cells (iHE); and
(ii) directing differentiation of iHE with a medium composition comprising a ROCK inhibitor, GMCSF, and MCSF;
thereby generating a population of induced immune regulatory cells comprising induced myeloid suppressive cells, wherein the induced myeloid suppressive cells are CD45+ and CD33+;
and wherein the cell population has enhanced therapeutic potential.
|