| CPC C07J 9/00 (2013.01) [A61P 1/00 (2018.01); A61P 19/02 (2018.01); A61P 25/16 (2018.01); A61P 25/18 (2018.01); A61P 25/20 (2018.01); A61P 25/22 (2018.01); A61P 25/28 (2018.01); A61P 35/00 (2018.01); C07J 17/00 (2013.01); C07J 43/003 (2013.01)] | 18 Claims |
|
1. A method for treating a CNS-related condition, comprising administering to a subject in need thereof an effective amount of a compound of Formula (I-66):
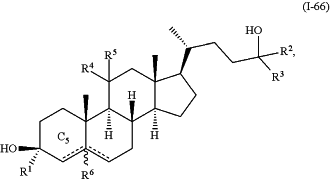 wherein:
R1 is substituted or unsubstituted C1-C6 alkyl;
R2 is substituted or unsubstituted aralkyl, substituted or unsubstituted heteroaralkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl;
R3 is hydrogen, substituted or unsubstituted C1-C6 alkyl, substituted or unsubstituted carbocyclyl, substituted or unsubstituted heterocyclyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl;
each of R4 and R5 is independently hydrogen, halo, or —ORC, wherein RC is hydrogen or unsubstituted or substituted C1-C3 alkyl, or
R4 and R5, together with the carbon atom to which they are attached form an oxo group;
R6 is absent or hydrogen; and
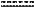 represents a single or double bond, wherein when one of represents a single or double bond, wherein when one of 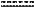 is a double bond, the other is a double bond, the other 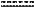 is a single bond; when both of is a single bond; when both of 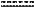 are single bonds, then R6 is hydrogen; and when one of are single bonds, then R6 is hydrogen; and when one of 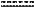 is a double bond, R6 is absent, or a pharmaceutically acceptable salt thereof; or a pharmaceutical composition comprising said compound of Formula (I-66), or a pharmaceutically acceptable salt thereof, and a pharmaceutically acceptable carrier; is a double bond, R6 is absent, or a pharmaceutically acceptable salt thereof; or a pharmaceutical composition comprising said compound of Formula (I-66), or a pharmaceutically acceptable salt thereof, and a pharmaceutically acceptable carrier;wherein the CNS-related condition is selected from the group consisting of schizophrenia, an autism spectrum disorder, Huntington's disease, Parkinson's disease, a seizure disorder, a mood disorder, stroke, a traumatic brain injury, a substance-related disorder, and a cognitive disorder.
|