| CPC C07F 7/0812 (2013.01) [A61P 1/04 (2018.01); A61P 17/06 (2018.01); A61P 19/02 (2018.01); A61P 25/00 (2018.01); A61P 29/00 (2018.01); A61P 43/00 (2018.01); C07C 237/22 (2013.01); C07D 205/04 (2013.01); C07D 207/12 (2013.01); C07D 207/26 (2013.01); C07D 209/18 (2013.01); C07D 211/62 (2013.01); C07D 213/64 (2013.01); C07D 231/12 (2013.01); C07D 231/56 (2013.01); C07D 233/72 (2013.01); C07D 237/14 (2013.01); C07D 239/36 (2013.01); C07D 239/54 (2013.01); C07D 261/08 (2013.01); C07D 261/12 (2013.01); C07D 271/10 (2013.01); C07D 295/14 (2013.01); C07D 309/06 (2013.01); C07D 309/08 (2013.01); C07D 333/38 (2013.01); C07D 403/06 (2013.01); C07D 405/12 (2013.01); C07D 413/12 (2013.01); C07D 487/04 (2013.01); C07F 7/081 (2013.01); C07F 7/10 (2013.01); C07F 7/30 (2013.01); C07K 5/06078 (2013.01); C07K 5/0812 (2013.01)] | 11 Claims |
|
1. A method for the treatment of inflammatory bowel disease (IBD), ulcerative colitis (UC), Crohn's disease (CD), rheumatoid arthritis, multiple sclerosis, uveitis, asthma, ankylosing spondylitis, or systemic lupus erythematosus (SLE), which comprises administering an effective amount of a compound represented by the following formula
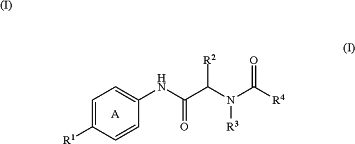 wherein
Ring A is a benzene ring optionally further substituted by 1 to 3 substituents selected from
(1) a halogen atom, and
(2) a cyano group;
R1 is
(1) a group represented by the formula: -Q(R1a)(R1b)(R1c)
wherein
Q is a carbon atom or a silicon atom, and
R1a, R1b and R1c are each independently a C1-6 alkyl group, or
(2) a neopentyl group;
R2 is
(1) a group represented by the formula:
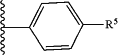 wherein
R5 is
(A) a C1-6 alkyl group optionally substituted by 1 to 3 C1-6 alkoxy groups, or
(B) a C1-6 alkoxy group,
(2) a bicyclic fused heterocyclic group selected from dihydrobenzofuryl and indazolyl, each optionally substituted by 1 to 3 C1-6 alkyl groups, or
(3) a group represented by the formula -L-Z1:
wherein
L is a bond; and
Z1 is
(A) a C3-10 cycloalkyl group optionally substituted by 1 to 3 halogen atoms, or
(B) tetrahydropyranyl;
R3 is
(1) a hydrogen atom, or
(2) a C1-6 alkyl group; and
R4 is
(1) a C1-6 alkyl group optionally substituted by 1 to 7 substituents selected from (a) a 3- to 8-membered monocyclic non-aromatic heterocyclic group selected from dihydropyrimidinyl, dihydropyridyl, dihydropyridazinyl, imidazolidinyl, tetrahydropyranyl, morpholinyl, piperidyl and tetrahydropyrimidinyl, each optionally substituted by 1 to 3 substituents selected from
(i) an oxo group, and
(ii) a C1-6 alkyl group,
(b) a 5- or 6-membered monocyclic aromatic heterocyclic group optionally substituted by 1 to 3 substituents selected from
(i) a C1-6 alkyl group, and
(ii) a hydroxy group,
(c) a 8- to 14-membered fused bicyclic aromatic heterocyclic group selected from indazolyl and benzimidazolyl,
(d) a halogen atom,
(e) a hydroxy group,
(f) a cyano group,
(g) a carboxy group,
(h) a C1-6 alkylsulfonyl group, and
(i) an amino group optionally mono- or di-substituted by C1-6 alkyl-carbonyl group(s),
(2) a 3- to 8-membered monocyclic non-aromatic heterocyclic group selected from pyrrolidinyl, oxazolidinyl, azetidinyl, piperidyl and tetrahydropyranyl, each optionally substituted by 1 to 3 substituents selected from
(a) an oxo group,
(b) a hydroxy group,
(c) a halogen atom, and
(d) a C1-6 alkyl-carbonyl group,
(3) a 5-membered monocyclic aromatic heterocyclic group optionally substituted by 1 to 3 hydroxy groups,
(4) a C3-10 cycloalkyl group optionally substituted by 1 to 3 halogen atoms, or
(5) an amino group optionally mono- or di-substituted by C1-6 alkyl group(s), provided that
α-(acetylamino)-N-[4-(1,1-dimethylethyl)phenyl]-cyclopentaneacetamide
is excluded,
or a salt thereof, to a mammal.
|