| CPC C07D 403/12 (2013.01) [C07D 403/14 (2013.01); C07D 405/14 (2013.01); C07D 413/12 (2013.01); C07D 413/14 (2013.01); C07D 487/04 (2013.01); C07D 491/107 (2013.01); C07D 498/04 (2013.01)] | 61 Claims |
|
1. A compound of Formula (I), or a pharmaceutically acceptable salt thereof, having the structure:
wherein:
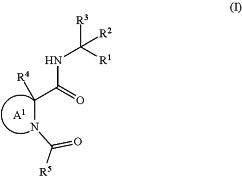 Ring A1 is selected from the group consisting of
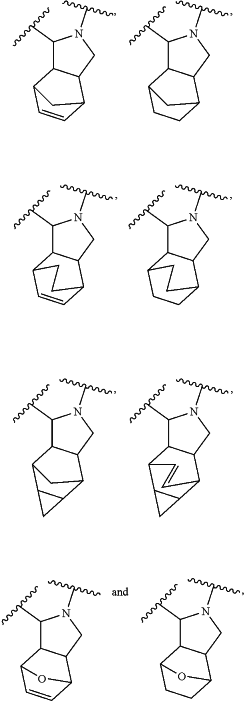 and wherein Ring A1 is optionally substituted with one or more moieties independently selected from the group consisting of ═O, ═CH2, deuterium, halogen, hydroxy, an unsubstituted C1-4 alkyl, an unsubstituted C1-4 haloalkyl, an unsubstituted C2-4 alkenyl and an unsubstituted or a substituted C3-6 monocyclic cycloalkyl, wherein the C3-6 monocyclic cycloalkyl is connected in a spiro-fashion;
R1 is selected from the group consisting of cyano, an unsubstituted or a substituted C2-5 alkynyl, an unsubstituted or a substituted acyl, an unsubstituted or a substituted ketoamide, —CH(OH)((P═O)(OR6)2) and —C(═O)CH2—O—((P═O)(OR7)2);
each R6 and each R7 re independently hydrogen, an unsubstituted C1-6 alkyl, an unsubstituted C2-6 alkenyl, an unsubstituted C1-6 haloalkyl, an unsubstituted or a substituted aryl or an unsubstituted or a substituted aryl(C1-4 alkyl);
R2 is hydrogen, deuterium or halogen;
R3 is an unsubstituted or a substituted monocyclic nitrogen-containing heterocyclyl(C1-4 alkyl), an unsubstituted or a substituted bicyclic nitrogen-containing heterocyclyl(C1-4 alkyl) or an unsubstituted or a substituted monocyclic nitrogen-containing heteroaryl(C1-4 alkyl);
R4 is hydrogen, deuterium or halogen;
R5 is
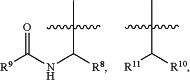 a substituted monocyclic C3-6 cycloalkyl or a substituted 4- to 6-membered monocyclic heterocyclyl, wherein the monocyclic C3-6 cycloalkyl and the 4- to 6-membered monocyclic heterocyclyl are independently substituted 1, 2 or 3 times with a moiety selected from the group consisting of deuterium, halogen, an unsubstituted C1-6 alkyl, an unsubstituted C1-6 haloalkyl and an unsubstituted C1-6 alkoxy, or independently substituted in a spiro-fashion by an unsubstituted or a substituted bicyclic cycloalkenyl or an unsubstituted or a substituted bicyclic heterocyclyl;
R8 and R10 are independently selected from the group consisting of an unsubstituted or a substituted C2-6 alkyl, an unsubstituted or a substituted C2-6 alkenyl, an unsubstituted or a substituted C2-6 alkynyl, an unsubstituted or a substituted monocyclic C3-6 cycloalkyl, an unsubstituted or a substituted bicyclic C5-8 cycloalkyl, an unsubstituted or a substituted monocyclic 4- to 6-membered heterocyclyl and an unsubstituted monocyclic C3-6 cycloalkyl(CH2)—,
wherein when the C2-6 alkyl is substituted, the C2-6 alkyl is substituted 1, 2, 3 or 4 times with a substituent independently selected from the group consisting of halogen, cyano, an unsubstituted or a substituted monocyclic C3-6 cycloalkyl, an unsubstituted C1-4 alkoxy and an unsubstituted C1-4 haloalkoxy, or the C2-6 alkyl is substituted 1 to 13 times with deuterium;
wherein when the C2-6 alkenyl, the C2-6 alkynyl, the monocyclic C3-6 cycloalkyl, the bicyclic C5-8 cycloalkyl and the monocyclic 4- to 6-membered heterocyclyl are substituted, the C2-6 alkenyl, the C2-6 alkynyl, the monocyclic C3-6 cycloalkyl, the bicyclic C5-8 cycloalkyl and the monocyclic 4- to 6-membered heterocyclyl are substituted 1, 2, 3 or 4 times with a substituent independently selected from the group consisting of halogen, an unsubstituted C1-4 alkyl, an unsubstituted C2-4 alkenyl, an unsubstituted C2-4 alkynyl, an unsubstituted C1-4 haloalkyl, an unsubstituted or a substituted monocyclic C3-6 cycloalkyl and an unsubstituted C1-4 alkoxy; and
R9 is selected from the group consisting of an unsubstituted or a substituted C1-6 alkyl, an unsubstituted or a substituted C1-6 haloalkyl, a substituted monocyclic C3-6 cycloalkyl, an unsubstituted or a substituted bicyclic C5-6 cycloalkyl, an unsubstituted or a substituted monocyclic heteroaryl and an unsubstituted or a substituted monocyclic heterocyclyl, wherein the substituted C1-6 alkyl is substituted 1 or 2 times with an unsubstituted C1-4 alkoxy, wherein the substituted monocyclic C3-6 cycloalkyl is substituted 1, 2, 3 or 4 times with a substituent independently selected from the group consisting of halogen, an unsubstituted C1-4 alkyl, an unsubstituted C1-4 alkoxy, an unsubstituted C1-4 haloalkyl and an unsubstituted monocyclic C3-6 cycloalkyl, and wherein the substituted C1-6 haloalkyl is substituted 1 or 2 times with an unsubstituted C1-4 alkoxy; and
R11 is selected from the group consisting of an optionally substituted monocyclic 4- to 6-membered heterocyclyl, —(NH)m-an optionally substituted 5- to 6-membered monocyclic heteroaryl, —O-an optionally substituted C1-6 alkyl, —O-an optionally substituted C3-8 cycloalkyl and —O-an optionally substituted C3-8 cycloalkyl(C1-4 alkyl), wherein m is 0 or 1.
|