| CPC B01L 3/502 (2013.01) [B01L 2200/0605 (2013.01); B01L 2400/0406 (2013.01)] | 29 Claims |
|
1. A method of separating and metering a plasma sample from a patient's liquid test sample for use within at least one diagnostic assay, the method comprising the steps of:
collecting a patient's liquid test sample into a sample device, the sample device comprising:
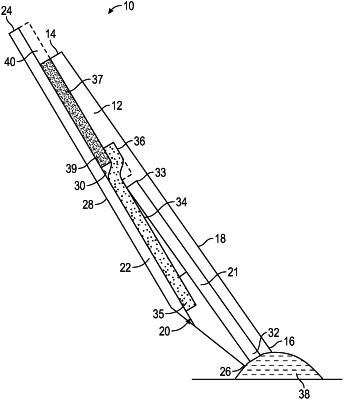
a top portion, the top portion comprising a first end, a second end, a top side, a bottom side, and a sample channel disposed between the top side and bottom side and having a first opening at the second end for collecting the patient's liquid test sample, the sample channel extending longitudinally from the second end to the first end of the top portion, wherein the first end has a second opening;
a bottom portion, the bottom portion comprising a first end, a second end, a top side, and a bottom side, wherein the bottom portion is secured to the top portion;
at least one red blood cell capture membrane, the at least one red blood cell capture membrane comprising a first end and a second end, wherein the second end of the red blood cell capture membrane is in substantially direct contact with the second opening of the sample channel; and
at least one plasma membrane comprising a first end and a second end, wherein the plasma membrane is configured and structured to hold a specific volume of plasma, wherein the first end of the plasma membrane is in substantially direct contact with the second end of the at least one red blood cell capture membrane, wherein the second end of the plasma membrane extends beyond the first end of the top portion thereby forming an exposed portion of the plasma membrane, wherein the exposed portion of the plasma membrane has a surface area that holds a predetermined volume of plasma, and wherein the exposed portion of the plasma membrane is isolatable from the remainder of the plasma membrane to meter and deliver a predetermined volume of patient plasma for performance of the one or more diagnostic assays;
transferring the patient's liquid test sample from the sample channel into the at least one red blood cell capture membrane such that any red blood cells contained within the patient's liquid test sample are separated and retained within the at least one red blood cell capture membrane, thereby forming a plasma sample;
allowing the plasma sample to flow from the at least one red blood cell capture membrane into the at least one plasma membrane whereby the plasma membrane is filled with the specific volume of plasma such that a predetermined volume of the plasma sample resides and is substantially contained within the exposed portion of the plasma membrane; and
pinching, separating, or cutting off the exposed portion of the plasma membrane to isolate the predetermined volume of the plasma sample for use in at least one diagnostic assay.
|