| CPC A61K 47/6803 (2017.08) [A61K 47/6811 (2017.08); A61K 47/6851 (2017.08); A61K 47/6855 (2017.08); A61K 47/6889 (2017.08); C07K 16/32 (2013.01)] | 28 Claims |
|
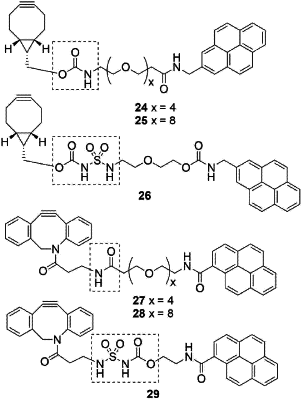
1. A bioconjugate, comprising a biomolecule B and a target molecule D, wherein the bioconjugate further comprises a group according to formula (1):
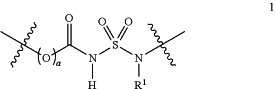 covalently linked to an alpha-end at one side of the formula (1) and an omega-end at the other side of the formula (1), the alpha-end comprising the biomolecule B and the omega-end comprising the target molecule D,
wherein:
the wavy lines indicate covalent linkages to the alpha-end and the omega-end,
the biomolecule B is selected from the group consisting of proteins polypeptides, peptides, glycans, nucleic acids, oligonucleotides, polysaccharides, oligosaccharides and enzymes;
the target molecule D is selected from the group consisting of colchicine, vinca alkaloids, anthracyclines camptothecins taxanes, calicheamycins, tubulysins, an inhibitory peptide, amanitin, deBouganin, duocarmycins, maytansines, auristatins ander pyrrolobenzodiazepines;
a is 0 or 1; and
R1 is selected from the group consisting of hydrogen, C1-C24 alkyl groups, C3-C24 cycloalkyl groups, C2-C24 (hetero)aryl groups, C3-C24 alkyl(hetero)aryl groups and C3-C24 (hetero)arylalkyl groups, the C1-C24 alkyl groups, C3-C24 cycloalkyl groups, C2-C24 (hetero)aryl groups, C3-C24 alkyl(hetero)aryl groups and C3-C24 (hetero)arylalkyl groups optionally substituted and optionally interrupted by one or more heteroatoms selected from O, S and NR3 wherein R3 is independently selected from the group consisting of hydrogen and C1-C4 alkyl groups, or R1 is a target molecule D, wherein the target molecule is optionally connected to N via a spacer moiety,
or a salt of the bioconjugate.
|