| CPC A61B 5/073 (2013.01) [A61B 5/4845 (2013.01); A61B 2562/02 (2013.01); A61B 2562/162 (2013.01); A61B 2562/164 (2013.01)] | 19 Claims |
|
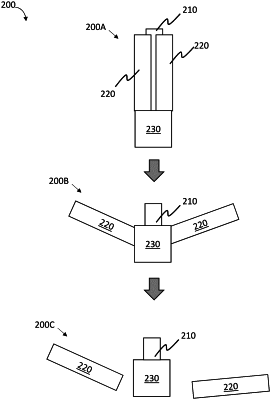
1. A residence article, comprising:
an alcohol sensor associated with a gastric residence article; and
one or more drug delivery modules;
wherein the gastric residence article is configured to be administered to a subject and to be retained at a location internal to the subject for at least 24 hours,
wherein the residence article has a first configuration sized and adapted for transesophageal administration to a subject,
wherein the residence article has a second configuration sized and adapted such that the residence article is retained in the stomach and prevented from passing through the pylorus, and
wherein the alcohol sensor, upon detection of one or more biophysical conditions in the location internal the subject, is configured to trigger release of at least one therapeutic agent from the one or more drug delivery modules to the location internal the subject.
|