| CPC G16H 20/10 (2018.01) [G06N 3/084 (2013.01); G06N 20/00 (2019.01); G16H 10/60 (2018.01); G16H 50/20 (2018.01); G16H 50/50 (2018.01); G16H 50/70 (2018.01)] | 30 Claims |
|
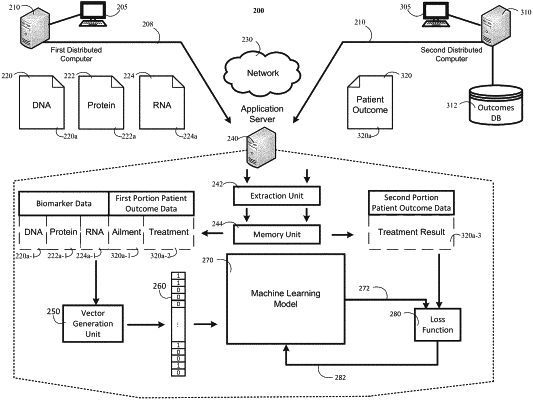
1. A system for selecting a treatment for a cancer in a first subject, the system comprising:
one or more computers; and
one or more memory devices storing instructions that, when executed by the one or more computers, cause the one or more computers to perform operations, the operations comprising:
obtaining, by the one or more computers, a plurality of copy numbers based on output data generated by a next generation sequencer, wherein the next generation sequencer generated the output data by sequencing a biological sample comprising cancer cells from the first subject, wherein the plurality of copy numbers include a copy number for each of the following groups of genes or proximate genomic regions thereto:
(a) Group 1 comprising a plurality of MYC, EP300, U2AF1, ASXL1, MAML2, CNTRL, WRN, and CDX2;
(b) Group 2 comprising a plurality of BCL9, PBX1, PRRX1, INHBA, and YWHAE;
(c) Group 3 comprising a plurality of BCL9, PBX1, GNAS, LHFPL6, CASP8, ASXL1, FH, CRKL, MLF1, TRRAP, AKT3, ACKR3, MSI2, PCM1, and MNX1;
(d) Group 4 comprising a plurality of BX1, GNAS, AURKA, CASP8, ASXL1, CRKL, MLF1, GAS7, MN1, SOX10, TCL1A, LMO1, BRD3, SMARCA4, PER1, PAX7, SBDS, SEPT5, PDGFB, AKT2, TERT, KEAP1, ETV6, TOP1, TLX3, COX6C, NFIB, ARFRP1, ARID1A, MAP2K4, NFKBIA, WWTR1, ZNF217, IL2, NSD3, CREB1, BRIP1, SDC4, EWSR1, FLT3, FLT1, FAS, CCNE1, RUNX1T1, and EZR; and
(e) Group 5 comprising a plurality of BCL9, PBX1, PRRX1, INHBA, YWHAE, GNAS, LHFPL6, FCRL4, BIRC3, AURKA, and HOXA11;
providing, by the one or more computers, input data as an input to a predictive model, wherein the input data includes the obtained plurality of copy numbers, wherein the predictive model includes multiple machine learning models, wherein each machine learning model is configured to process the obtained copy numbers for one of Group 1, Group 2, Group 3, Group 4, and Group 5;
processing, by the one or more computers, the input data that includes the obtained copy numbers for each group through one of the multiple machine learning models, wherein the processing includes:
processing, by the one or more computers, the obtained copy numbers for Group 1 through a first machine learning model of the multiple machine learning models to generate first data indicating whether the first subject is likely to benefit from a treatment that includes platinum therapy, wherein the first machine learning model has been trained to generate output data indicative of a likely benefit from a treatment that includes platinum therapy based on processing input data that includes a copy number for a group of genes comprising MYC, EP300, U2AF1, ASXL1, MAML2, CNTRL, WRN, and CDX2,
processing, by the one or more computers, obtained copy numbers for Group 2 through a second machine learning model of the multiple machine learning models to generate second data indicating whether the first subject is likely to benefit from the treatment that includes platinum therapy, wherein the second machine learning model has been trained to generate output data indicative of a likely benefit from a treatment that includes platinum therapy based on processing input data that includes a copy number for a group of genes comprising BCL9, PBX1, PRRX1, INHBA, and YWHAE,
processing, by the one or more computers, the obtained copy numbers for Group 3 through a third machine learning model of the multiple machine learning models to generate third data indicating whether the first subject is likely to benefit from the treatment that includes platinum therapy, wherein the third machine learning model has been trained to generate output data indicative of a likely benefit from a treatment that includes platinum therapy based on processing input data that includes a copy number for a group of genes comprising BCL9, PBX1, GNAS, LHFPL6, CASP8, ASXL1, FH, CRKL, MLF1, TRRAP, AKT3, ACKR3, MSI2, PCM1, and MNX1,
processing, by the one or more computers, the obtained copy numbers for Group 4 through a fourth machine learning model of the multiple machine learning models to generate fourth data indicating whether the first subject is likely to benefit from the treatment that includes platinum therapy, wherein the fourth machine learning model has been trained to generate output data indicative of a likely benefit from a treatment that includes platinum therapy based on processing input data that includes a copy number for a group of genes comprising BX1, GNAS, AURKA, CASP8, ASXL1, CRKL, MLF1, GAS7, MN1, SOX10, TCL1A, LMO1, BRD3, SMARCA4, PER1, PAX7, SBDS, SEPT5, PDGFB, AKT2, TERT, KEAP1, ETV6, TOP1, TLX3, COX6C, NFIB, ARFRP1, ARID1A, MAP2K4, NFKBIA, WWTR1, ZNF217, IL2, NSD3, CREB1, BRIP1, SDC4, EWSR1, FLT3, FLT1, FAS, CCNE1, RUNX1T1, and EZR, and
processing, by the one or more computers, the obtained copy numbers for Group 5 through a fifth machine learning model of the multiple machine learning models to generate fifth data indicating whether the first subject is likely to benefit from the treatment that includes platinum therapy, wherein the fifth machine learning model has been trained to generate output data indicative of a likely benefit from a treatment that includes platinum therapy based on processing input data that includes a copy number for a group of genes comprising BCL9, PBX1, PRRX1, INHBA, YWHAE, GNAS, LHFPL6, FCRL4, BIRC3, AURKA, and HOXA11;
determining, by the one or more computers and based on the first data, the second data, the third data, and the fifth data, whether a majority of the multiple machine learning models indicate that the first subject is likely to benefit from the treatment that includes platinum therapy;
based on a determination that a majority of the machine learning models indicate that the first subject is likely to benefit from the treatment that includes platinum therapy, identifying, by the one or more computers, data that identifies the treatment that includes platinum therapy; and
providing, by the one or more computers, output that identifies the treatment that includes platinum therapy.
|