| CPC G06F 11/3466 (2013.01) [A61B 5/7275 (2013.01); G06F 11/3438 (2013.01); G06N 20/00 (2019.01)] | 24 Claims |
|
1. A method performed by one or more computers, the method comprising:
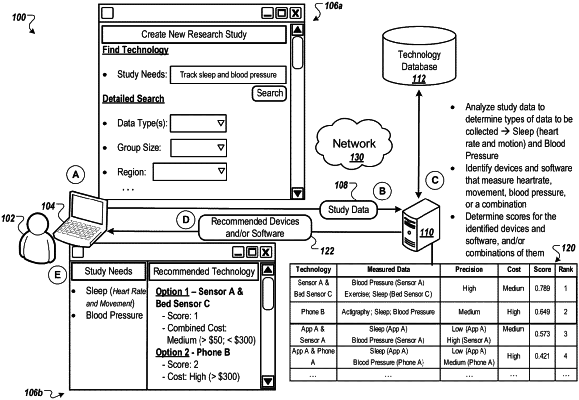
providing, by the one or more computers, a technology database that indicates characteristics of each of a plurality of different types of devices or software items;
identifying, by the one or more computers, records that indicate (i) usage of the different types of devices or software items by different individuals to monitor health of the individuals or to provide medical treatment to the individuals and (ii) results of the individuals using the different types of devices or software items;
identifying, by the one or more computers, a plurality of different types of health monitoring or medical treatment indicated by the records;
for each of the different types of devices or software items, validating, by the one or more computers, the type of device or software item to be used in one or more of the identified types of health monitoring or medical treatment based on the identified records, wherein validating the type of device or software item for a type of health monitoring or medical treatment involves determining that the results that the records indicate for use of the type of device or software item satisfy one or more validation criteria for the type of health monitoring or medical treatment;
updating, by the one or more computers, the technology database to store validation information specifying the types of health monitoring or medical treatment for which the respective devices or software items are validated;
receiving, by the one or more computers, attribute data indicating attributes of an individual; and
using, by the one or more computers, the attribute data for the individual and the validation information in the updated technology database to select one or more of the types of devices or software items for at least one of monitoring health of the individual or providing medical treatment to the individual.
|