| CPC C07D 491/18 (2013.01) [A61K 9/0073 (2013.01); A61K 9/7092 (2013.01); A61K 31/485 (2013.01); A61P 25/04 (2018.01); A61P 25/24 (2018.01); C07D 471/18 (2013.01); C07D 495/18 (2013.01); A61K 9/0002 (2013.01); A61K 47/38 (2013.01)] | 27 Claims |
|
1. A compound having the structure:
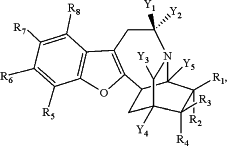 wherein
Y1 is H, -(alkyl), -(alkenyl), -(alkynyl),—(C3-C5 cycloalkyl), -(haloalkyl), -(alkyl)-O-(alkyl) or -(alkyl)-(C3-C5cycloalkyl);
Y2 is H, -(alkyl), -(alkenyl), -(alkynyl), —(C3-C5cycloalkyl), -(haloalkyl), -(alkyl)-O-(alkyl) or -(alkyl)-(C3-C5cycloalkyl);
Y3 is H, -(alkyl), -(alkenyl), -(alkynyl), —(C3-C5cycloalkyl), -(haloalkyl), -(alkyl)-O-(alkyl) or -(alkyl)-(C3-C5cycloalkyl);
Y4 is H, -(alkyl), -(alkenyl), -(alkynyl), —(C3-C5cycloalkyl), -(haloalkyl), -(alkyl)-O-(alkyl) or -(alkyl)-(C3-C5cycloalkyl);
Y5 is H, -(alkyl), -(alkenyl), -(alkynyl), —(C3-C5cycloalkyl), -(haloalkyl), -(alkyl)-O-(alkyl) or -(alkyl)-(C3-C5cycloalkyl);
R1, R2, R3 and R4 are each independently —H, -(alkyl), -(alkenyl), -(alkynyl), -(haloalkyl), —(C3-C5 cycloalkyl), -(phenyl), -(heteroalkyl), -(hydroxyalkyl), benzyl, -(alkyl)-(C3-C5 cycloalkyl), -(alkyl)-OH, -(alkyl)-O-(alkyl), —OH, —NH2, —OAc, —CN, OCF3, halogen, C(O)—NH2, —C(O)—NH-(alkyl), C(O)—NH-(phenyl), —O-alkyl, —O-alkenyl, —O-alkynyl, —O-phenyl, —NH-alkyl, —NH— alkenyl, —NH-alkynyl, —NH-phenyl, or —C(O)—N(alkyl)2; and
R5, R6, R7, R8 are each independently —H, —CN, —CF3, —OCF3, -(alkyl), -(alkenyl), -(alkynyl), -(phenyl), -(heteroalkyl), -(hydroxyalkyl), —NH2, —NH-(alkyl), —NH-(alkenyl), —NH-(alkynyl), —NH-(phenyl), —OH, —OAc, —CO2H, —CO2-(alkyl), —O—C(O)(alkyl), —O-(alkyl), —O-(alkenyl), —O-(alkynyl), —O-(phenyl), C(O)—NH2, C(O)—NH-(alkyl), or C(O)—NH-(phenyl),
or an enantiomer, racemic mixture, or a pharmaceutically acceptable salt thereof.
|