| CPC C07D 487/04 (2013.01) [A61K 45/06 (2013.01)] | 18 Claims |
|
1. A compound of formula (I), or pharmaceutically acceptable salts, stereoisomers, solvates, hydrates, crystal forms, prodrugs or isotopic variants thereof:
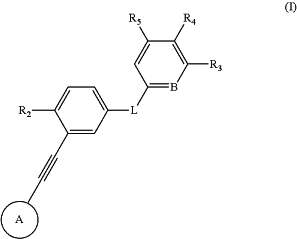 wherein,
ring A is:
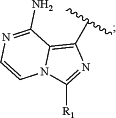 R1 is selected from C1-6 alkyl, C3-7 cycloalkyl, 3- to 7-membered heterocyclyl, C6-10 aryl or 5- to 10-membered heteroaryl, wherein the groups are optionally substituted by one or more R1a;
R2 is H or C1-6 alkyl;
L is selected from —C(O)N(R1b)—, —N(R1b)C(O)— or —N(R1b)C(O)N(R1b)—;
B is selected from CH or N;
R3 is selected from C1-6 alkyl or C1-6 haloalkyl;
R4 and R5 are selected from:
1) R5 is H, R4 is selected from H, C1-6 alkyl, —C0-2 alkylene-C3-7 cycloalkyl, —C0-2 alkylene-3- to 7-membered heterocyclyl or —C0-2 alkylene-NR1cR2c, wherein the groups are optionally substituted by one or more R1d; or,
2) R4 is H, R5 is selected from H or 5- to 6-membered heteroaryl containing one or more N, O or S heteroatoms, wherein the 5- to 6-membered heteroaryl ring is optionally substituted by one or more R1d;
each R1a is independently selected from H, halogen, oxo, —C0-6 alkylene-ORa, —C0-6 alkylene-NRbRc, —C0-6 alkylene-C(O)Ra, —C0-6 alkylene-C(O)ORa, —C0-6 alkylene-C(O)NRbRc, C1-6 alkyl, C1-6 haloalkyl, —C0-6 alkylene-C3-6 cycloalkyl or —C0-6 alkylene-3- to 7-membered heterocyclyl; or two or more R1a together with the atom to which they attached form a C3-7 cycloalkyl or 3- to 7-membered heterocyclyl;
each R1b is independently selected from H, —C0-6 alkylene-ORa, —C0-6 alkylene-NRbRc, —C0-6 alkylene-C(O)Ra, —C0-6 alkylene-C(O)ORa, —C0-6 alkylene-C(O)NRbRc, C1-6 alkyl, C1-6 haloalkyl, —C0-6 alkylene-C3-6 cycloalkyl or —C0-6 alkylene-3- to 7-membered heterocyclyl; or two or more R1b together with the atom to which they attached form a C3-7 cycloalkyl or 3- to 7-membered heterocyclyl;
R1c and R2 are each independently selected from H, halogen, —C0-6 alkylene-CN, —C0-6 alkylene-ORa, —C0-6 alkylene-SRa, —C0-6 alkylene-NRbRc, —C0-6 alkylene-C(O)Ra, —C0-6 alkylene-C(O)ORa, —C0-6 alkylene-C(O)NRbRc, C1-6 alkyl, C1-6 haloalkyl, C2-6 alkenyl, C2-6 alkynyl, —C0-6 alkylene-C3-6 cycloalkyl, —C0-6 alkylene-3- to 7-membered heterocyclyl, —C0-6 alkylene-C6-10 aryl or —C0-6 alkylene-5- to 10-membered heteroaryl; or, R1c, R2 together with the nitrogen atom to which they attached form a C3-7 cycloalkyl or 3- to 7-membered heterocyclyl;
each R1d is independently selected from H, halogen, —C0-6 alkylene-CN, —C0-6 alkylene-ORa, —C0-6 alkylene-SRa, —C0-6 alkylene-NRbRc, —C0-6 alkylene-C(O)Ra, —C0-6 alkylene-C(O)ORa, —C0-6 alkylene-C(O)NRbRc, C1-6 alkyl, C1-6 haloalkyl, C2-6 alkenyl, C2-6 alkynyl, —C0-6 alkylene-C3-6 cycloalkyl, —C0-6 alkylene-3- to 7-membered heterocyclyl, —C0-6 alkylene-C6-10 aryl or —C0-6 alkylene-5- to 10-membered heteroaryl; or two or more R1d together with the atom to which they attached form a C3-7 cycloalkyl or 3- to 7-membered heterocyclyl;
Ra, Rb and Rc are independently selected from H, C1-6 alkyl, C1-6 haloalkyl, C3-7 cycloalkyl, 3- to 7-membered heterocyclyl, C6-10 aryl or 5- to 10-membered heteroaryl.
|