| CPC A61K 31/4025 (2013.01) [A61K 31/437 (2013.01); A61K 31/506 (2013.01); A61K 31/519 (2013.01); A61K 45/06 (2013.01)] | 15 Claims |
|
1. A pharmaceutical kit for the treatment of melanoma, the kit comprising a CDK (cyclin dependent kinase) inhibitor of Formula I, or a pharmaceutically acceptable salt thereof;
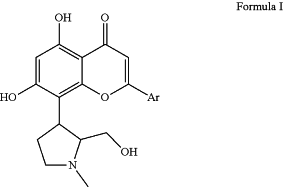 wherein Ar is 2 chloro-4-trifluoromethylphenyl; and a package insert comprising instructions directing the use of the CDK inhibitor in a combined treatment with a therapeutically effective amount of at least one anticancer agent selected from vemurafenib, dabrafenib, and trametinib.
|