| CPC A61F 2/856 (2013.01) [A61F 2/07 (2013.01); A61F 2/2418 (2013.01); A61F 2/82 (2013.01); A61F 2/962 (2013.01); A61F 2/24 (2013.01); A61F 2/86 (2013.01); A61F 2/89 (2013.01); A61F 2/90 (2013.01); A61F 2002/061 (2013.01); A61F 2002/067 (2013.01); A61F 2210/00 (2013.01); A61F 2210/0014 (2013.01); A61F 2210/0057 (2013.01); A61F 2220/0008 (2013.01); A61F 2220/0016 (2013.01); A61F 2220/0025 (2013.01); A61F 2220/0058 (2013.01); A61F 2220/0075 (2013.01); A61F 2230/001 (2013.01); A61F 2230/0034 (2013.01); A61F 2230/0067 (2013.01); A61F 2250/0039 (2013.01); A61F 2250/0048 (2013.01); A61F 2250/0069 (2013.01)] | 22 Claims |
|
1. A transcatheter heart valve assembly comprising:
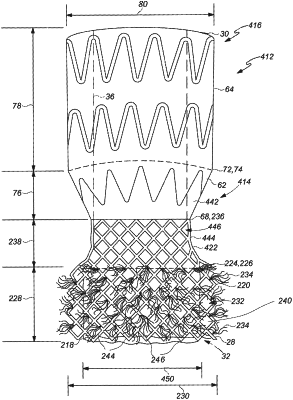
a frame extending around prosthetic heart valve leaflets,
wherein the frame is secured to a covering material by a plurality of stitches,
a sealing material positioned externally to the frame for providing sealing between the frame and a patient's anatomical wall to prevent paravalvular leaks, wherein the sealing material includes a plurality of outwardly extending arcuate fibers that extend outwardly away from the frame, wherein the sealing material is attached to the frame and extends over at least a portion of the frame,
wherein the sealing material is free of a graft covering between the fibers and an outside surface of the frame when the valve assembly is in an undeployed state,
wherein expansion of the valve assembly presses the sealing material against native leaflets of an aorta of the patient,
wherein the valve assembly has a radially compressed orientation and a radially expanded orientation,
wherein the valve assembly is sized and shaped to be delivered endovascularly through a femoral artery of the patient.
|