| CPC A61K 31/192 (2013.01) [A61K 9/0073 (2013.01); A61K 9/1272 (2013.01); A61K 47/28 (2013.01); A61P 11/00 (2018.01)] | 20 Claims |
|
1. A method of treating a respiratory disease, comprising the steps of administering a pharmaceutical composition comprising:
one or more liposome, said liposome comprising:
(a) an external lipid bilayer, comprising at least one vesicle-forming phospholipid; and
(b) an internal aqueous medium, comprising treprostinil and a salt to provide a pH gradient between the internal aqueous medium,
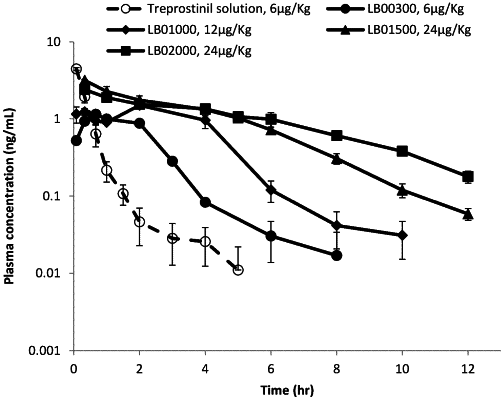
wherein the weight ratio of treprostinil to the at least one vesicle-forming phospholipid (T/P ratio) is equal to or higher than about 0.035 and about less than 60% of the treprostinil is released within 2 hours after the administration of the pharmaceutical composition and more than 80% of the treprostinil is released more than 2 hours to about 72 hours after the administration of the pharmaceutical composition.
|