| CPC C07C 227/12 (2013.01) [B01J 19/0093 (2013.01); C07C 227/08 (2013.01); B01J 2219/0086 (2013.01); B01J 2219/00867 (2013.01); B01J 2219/00889 (2013.01)] | 9 Claims |
|
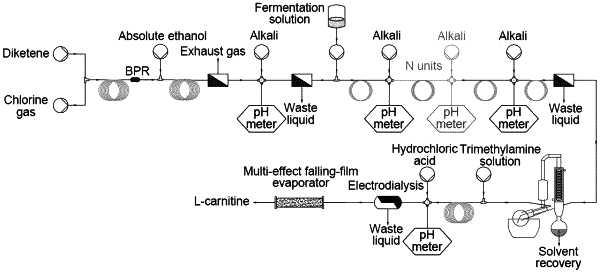
1. A full continuous-flow preparation method of L-carnitine using a micro-reaction system, the micro-reaction system comprising a first micromixer, a first microchannel reactor, a second micromixer, a second microchannel reactor, a third micromixer, a third microchannel reactor, a fourth micromixer and a fourth microchannel reactor communicated in sequence; and the method comprising:
(S1) respectively transporting chlorine gas and a diketene reaction liquid to the first micromixer for mixing, and allowing the reaction mixture in the first micromixer to flow into the first microchannel reactor followed by a continuous chlorination reaction;
(S2) transporting the reaction mixture flowing out from the first microchannel reactor and an alcohol solvent into the second micromixer and the second microchannel reactor in sequence fora continuous esterification reaction;
(S3) neutralizing the reaction mixture flowing out from the second microchannel reactor with a first alkali followed by a continuous extraction and separation to collect a first organic phase; simultaneously transporting the first organic phase and an aqueous solution of a reductase to the third micromixer and the third microchannel reactor fora continuous reduction reaction;
(S4) removing the reductase from the reaction mixture flowing out from the third microchannel reactor via an extraction separator followed by a continuous extraction and separation to obtain a second organic phase; and concentrating the second organic phase to obtain a concentrated liquid;
(S5) simultaneously transporting the concentrated liquid obtained in step (S4) and a trimethylamine solution to the fourth micromixer and the fourth microchannel reactor in sequence fora continuous substitution and hydrolysis reaction; and
(S6) neutralizing the reaction mixture flowing out from the fourth microchannel reactor with a second alkali followed by feeding the neutralized reaction mixture into a demineralization device and a concentration device to obtain the L-carnitine.
|