| CPC C07K 16/2851 (2013.01) [C07K 2317/24 (2013.01); C07K 2317/565 (2013.01); C07K 2317/76 (2013.01); C07K 2317/92 (2013.01)] | 19 Claims |
|
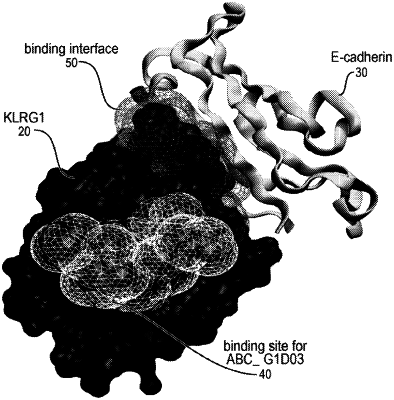
1. A method of treating a disorder associated with excess or unwanted killer cell lectin-like receptor G1 (KLRG1) expressing T cells in a subject in need thereof, comprising:
delivering to the subject a therapeutically effective amount of an antibody, or a fragment thereof, that specifically binds to an extracellular domain of KLRG1 without interfering with binding by E-cadherin, N-cadherin, or R-cadherin to the extracellular domain of KLRG1 and comprises a heavy chain variable region comprising three heavy chain complementarity determining regions (CDR-H1, CDR-H2 and CDR-H3) and a light chain variable region comprising three light chain complementary determining regions (CDR-L1, CDR-L2 and CDR-L3),
wherein said antibody, or fragment thereof, comprises
i) SEQ ID NO:16 (CDR-H1), SEQ ID NO:17 (CDR-H2), SEQ ID NO:18 (CDR-H3), SEQ ID NO:19 (CRD-L1), SEQ ID NO:20 (CDR-L2), and SEQ ID NO:21 (CDR-L3);
ii) SEQ ID NO:22 (CDR-H1), SEQ ID NO:23 (CDR-H2), SEQ ID NO:24 (CDR-H3), SEQ ID NO:25 (CRD-L1), SEQ ID NO:26 (CDR-L2), and SEQ ID NO:27 (CDR-L3);
iii) SEQ ID NO:28 (CDR-H1), SEQ ID NO:29 (CDR-H2), SEQ ID NO:30 (CDR-H3), SEQ ID NO:31 (CRD-L1), SEQ ID NO:32 (CDR-L2), and SEQ ID NO:33 (CDR-L3);
iv) SEQ ID NO:34 (CDR-H1), SEQ ID NO:35 (CDR-H2), SEQ ID NO:36 (CDR-H3), SEQ ID NO:37 (CRD-L1), SEQ ID NO:38 (CDR-L2), and SEQ ID NO:39 (CDR-L3);
v) SEQ ID NO:40 (CDR-H1), SEQ ID NO:41 (CDR-H2), SEQ ID NO:42 (CDR-H3), SEQ ID NO:43 (CRD-L1), SEQ ID NO:44 (CDR-L2), and SEQ ID NO:45 (CDR-L3); or
vi) SEQ ID NO:46 (CDR-H1), SEQ ID NO:47 (CDR-H2), SEQ ID NO:48 (CDR-H3), SEQ ID NO:49 (CRD-L1), SEQ ID NO:50 (CDR-L2), and SEQ ID NO:51 (CDR-L3);
and wherein the delivery of the therapeutically effective amount of the antibody, or fragment thereof, depletes the excess or unwanted KLRG1 expressing T cells in the subject.
|