| CPC C07D 487/14 (2013.01) [A61K 45/06 (2013.01); C07B 2200/13 (2013.01)] | 1 Claim |
|
1. A pharmaceutical composition comprising a pharmaceutically acceptable excipient for solid dosage delivery and between about 10 mg and about 1,000 mg of an isolated crystalline Form B of the di-HCl salt of structure:
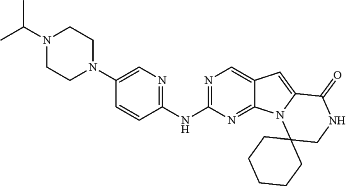 characterized by an X-ray powder diffraction (XRPD) pattern comprising at least three 2θ values selected from 6.5±0.2°, 9.5±0.4°, 14.0±0.2°, 14.4±0.2°, 18.1±0.2°, 19.3±0.2°, 19.9±0.2°, and 22.4±0.2°.
|