| CPC C07D 487/04 (2013.01) [B01J 21/04 (2013.01); B82Y 30/00 (2013.01)] | 9 Claims |
|
1. A process for preparing a compound of Formula (II):
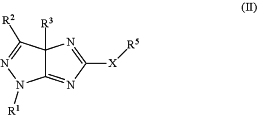 wherein:
R1 is H, halogen, alkyl, cycloalkyl, aryl, or heteroaryl;
R2 is H, halogen, alkyl, cycloalkyl, aryl, or heteroaryl;
R3 is H or C1-C3 alkyl; and
(i) X is —NH—; and
R5 is C(S)NH2; or
(ii) X is —S—; and
R5 is CH2C(O)OCH2CH3;
with the proviso that R2 is not pyrimidinyl or triazinyl;
wherein the process comprises the following steps:
(1) dissolving chitosan in acetic acid to obtain a chitosan solution;
(2) adjusting the pH of the chitosan solution formed in step (1) above to a pH in the range of 6 to 7;
(3) adding an aqueous suspension of aluminum oxide nanoparticles portion-wise to the chitosan solution formed in step (2) above, followed by stirring to form an aqueous mixture containing the chitosan solution and aluminum oxide nanoparticles;
(4) solution casting the aqueous mixture containing chitosan and aluminum oxide nanoparticles formed in step (3) above onto a carrier substrate, followed by drying to form a chitosan-alumina nanocomposite film;
wherein the chitosan-alumina nanocomposite film comprises aluminum oxide dispersed in a chitosan matrix;
wherein the chitosan matrix envelopes the aluminum oxide nanoparticles through N—H and O—H hydrogen bonding; and
wherein the chitosan-alumina nanocomposite film is a heterogeneous base catalyst;
(5) reacting a compound of Formula (III):
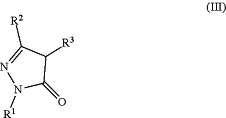 wherein:
R1 is H, halogen, alkyl, cycloalkyl, aryl, or heteroaryl;
R2 is H, halogen, alkyl, cycloalkyl, aryl, or heteroaryl; and
R3 is H or C1-C3 alkyl;
with the proviso that R2 is not pyrimidinyl or triazinyl;
with a compound of Formula (IV):
 wherein:
R4 is H, alkyl, cycloalkyl, aryl, or heteroaryl;
in the presence of ethanol and the chitosan-alumina nanocomposite film formed in step (4) above [10-20 wt % based on the weight of the compound of Formula (III)], to form a reaction mixture comprising ethanol and the heterogeneous base chitosan-alumina nanocomposite film catalyst;
(6) filtering the reaction mixture formed in step (5) above to remove the heterogeneous base chitosan-alumina nanocomposite film catalyst, followed by collecting a filtrate containing ethanol;
(7) optionally recycling the heterogeneous base chitosan-alumina nanocomposite film catalyst removed in step (5) above;
(8) evaporating ethanol from the filtrate collected in step (6) above, to precipitate a crude compound of Formula (I):
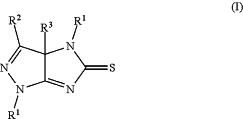 wherein:
R1 is H, halogen, alkyl, cycloalkyl, aryl, or heteroaryl;
R2 is H, halogen, alkyl, cycloalkyl, aryl, or heteroaryl;
R3 is H or C1-C3 alkyl; and
R4 is H, alkyl, cycloalkyl, aryl, or heteroaryl;
with the proviso that R2 is not pyrimidinyl or triazinyl;
(9) reacting the crude compound of Formula (I) precipitated in step (8) above with thiourea, H2N—C(S)—NH2, or ethyl chloroacetate, ClCH2C(O)OCH2CH3, in the presence of dimethylformamide (DMF) or dry acetone and the chitosan-alumina nanocomposite film formed in step (4) above [10-20 wt % based on the weight of the compound of Formula (I)], to form a reaction mixture comprising dimethylformamide (DMF) or acetone and the heterogeneous base chitosan-alumina nanocomposite film catalyst;
(10) filtering the reaction mixture formed in step (9) above to remove the heterogeneous base chitosan-alumina nanocomposite film catalyst, followed by collecting a filtrate containing dimethylformamide (DMF) or acetone;
(11) evaporating dimethylformamide (DMF) or acetone from the filtrate collected in step (10) above, to precipitate the crude compound of Formula (II) above; and (12) recrystallizing the crude compound of Formula (II) precipitated in step (11) above from ethanol, to form the compound of Formula (II):
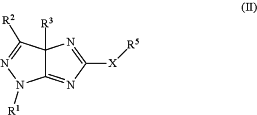 wherein:
R1 is H, halogen, alkyl, cycloalkyl, aryl, or heteroaryl;
R2 is H, halogen, alkyl, cycloalkyl, aryl, or heteroaryl;
R3 is H or C1-C3 alkyl; and
(i) X is —NH—; and
R5 is C(S) NH2; or
(ii) X is —S—; and
R5 is CH2C(O)OCH2CH3;
with the proviso that R2 is not pyrimidinyl or triazinyl.
|