| CPC A61N 1/39046 (2017.08) [A61N 1/3925 (2013.01); A61N 1/3968 (2013.01); A61N 1/3975 (2013.01); A61N 1/3993 (2013.01)] | 25 Claims |
|
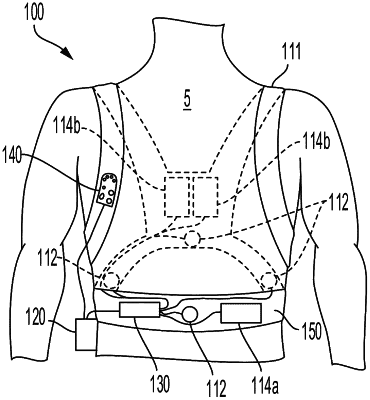
1. A serviceable wearable cardiac treatment device for continuous extended use by an ambulatory patient, comprising:
a garment configured to dispose therein a plurality of ECG sensing and therapy electrodes in continuous extended contact with the patient, and
a device controller configured to be in separable electrical communication with the plurality of ECG sensing and therapy electrodes in the garment, the device controller comprising
an impact-resistant energy core; comprising
a frame, and
at least one capacitor permanently bonded to the frame, the at least one capacitor configured to hold electrical charge sufficient to treat a cardiac arrhythmia, wherein the frame comprises a pocket configured to receive the at least one capacitor therein and a compound is disposed within the pocket to at least partially encapsulate the at least one capacitor thereby immovably binding the at least one capacitor to the frame to form a unitary mass;
a critical function circuit board comprising at least one critical function processor and critical function circuitry in communication with the at least one critical function processor, wherein the critical function circuit board is in electrical communication with the at least one capacitor and configured to control critical operations of the device controller; and
a non-critical function circuit board comprising at least one non-critical function processor and non-critical function circuitry in communication with the at least one non-critical function processor, wherein the non-critical function circuit board is configured to control non-critical operations of the device controller;
wherein the critical function circuit board is configured to control the critical operations of the device controller regardless of operability of the non-critical function circuit board.
|