| CPC C07K 16/40 (2013.01) [C07K 16/10 (2013.01); C07K 16/283 (2013.01); A61K 2039/505 (2013.01); C07K 2317/565 (2013.01); C07K 2317/569 (2013.01); C07K 2317/73 (2013.01); C07K 2317/732 (2013.01); C07K 2317/92 (2013.01)] | 18 Claims |
|
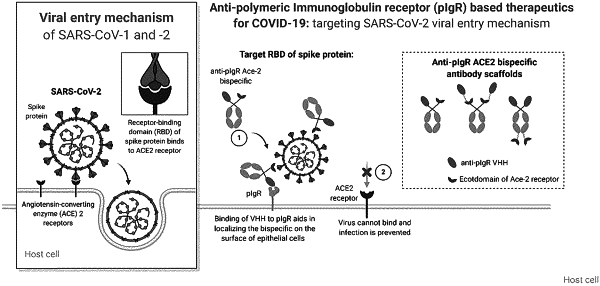
1. A multispecific molecule comprising: (a) a first binding domain that specifically binds to polymeric immunoglobulin receptor (pIgR), and (b) a second binding domain that specifically binds to SARS-COV-2, wherein the first binding domain comprises a single-domain antibody (VHH) comprising:
(a) a CDR1 comprising the amino acid sequence of SEQ ID NO:1, a CDR2 comprising the amino acid sequence of SEQ ID NO:2, and a CDR3 comprising the amino acid sequence of SEQ ID NO:3;
(b) a CDR1 comprising the amino acid sequence of SEQ ID NO:4, a CDR2 comprising the amino acid sequence of SEQ ID NO:5, and a CDR3 comprising the amino acid sequence of SEQ ID NO:6;
(c) a CDR1 comprising the amino acid sequence of SEQ ID NO:7, a CDR2 comprising the amino acid sequence of SEQ ID NO:8, and a CDR3 comprising the amino acid sequence of SEQ ID NO:9;
(d) a CDR1 comprising the amino acid sequence of SEQ ID NO: 10, a CDR2 comprising the amino acid sequence of SEQ ID NO: 11, and a CDR3 comprising the amino acid sequence of SEQ ID NO:12;
(e) a CDR1 comprising the amino acid sequence of SEQ ID NO:13, a CDR2 comprising the amino acid sequence of SEQ ID NO:14, and a CDR3 comprising the amino acid sequence of SEQ ID NO:15;
(f) a CDR1 comprising the amino acid sequence of SEQ ID NO:17, a CDR2 comprising the amino acid sequence of SEQ ID NO: 18, and a CDR3 comprising the amino acid sequence of SEQ ID NO:19;
(g) a CDR1 comprising the amino acid sequence of SEQ ID NO:20, a CDR2 comprising the amino acid sequence of SEQ ID NO:21, and a CDR3 comprising the amino acid sequence of SEQ ID NO:22;
(h) a CDR1 comprising the amino acid sequence of SEQ ID NO:23, a CDR2 comprising the amino acid sequence of SEQ ID NO:24, and a CDR3 comprising the amino acid sequence of SEQ ID NO:25;
(i) a CDR1 comprising the amino acid sequence of SEQ ID NO:26, a CDR2 comprising the amino acid sequence of SEQ ID NO:27, and a CDR3 comprising the amino acid sequence of SEQ ID NO:28; or
(j) a CDR1 comprising the amino acid sequence of SEQ ID NO:29, a CDR2 comprising the amino acid sequence of SEQ ID NO:30, and a CDR3 comprising the amino acid sequence of SEQ ID NO:31;
and wherein the second binding domain comprises a peptide comprising:
(a) angiotensin-converting enzyme 2 (ACE2);
comprising the amino acid sequence of SEQ ID NO: 194;
(b) the extracellular domain of ACE2;
comprising the amino acid sequence of SEQ ID NO: 134; or
(c) a truncated extracellular domain of ACE2;
comprising the amino acid sequence of SEQ ID NO: 120 or the amino acid sequence of SEQ ID NO: 121.
|