| CPC C07D 487/04 (2013.01) [C07B 2200/13 (2013.01)] | 21 Claims |
|
1. A crystalline salt or co-crystal of 2′,6-difluoro-5′-[3-(1-hydroxy-1-methylethyl)imidazo[1,2-b][1,2,4]triazin-7-yl]biphenyl-2-carbonitrile with p-toluenesulfonic acid of the following formula:
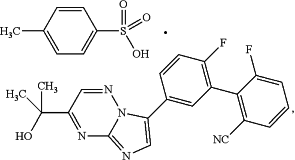 wherein the crystalline salt or co-crystal is Form A; and
wherein the crystalline salt or co-crystal is characterized by an X-ray powder diffraction pattern comprising at least three characteristic peaks at angles (°2θ) selected from 7.0°±0.2 °2θ, 12.4°±0.2 °2θ, 12.6°±0.2 °2θ, 13.0°±0.2 °2θ, 14.1°±0.2 °2θ, 15.4°±0.2 °2θ, 15.7°±0.2 °2θ, 16.3°±0.2 °2θ, 17.5°±0.2 °2θ, 18.3°±0.2 °2θ, 19.0°±0.2 °2θ, 21.0°±0.2 °2θ, 22.3°±0.2 °2θ, 23.0°±0.2 °2θ, and 24.9°±0.2 °2θ, when measured using the parameters described in Table 1.
|