| CPC C07D 471/04 (2013.01) | 13 Claims |
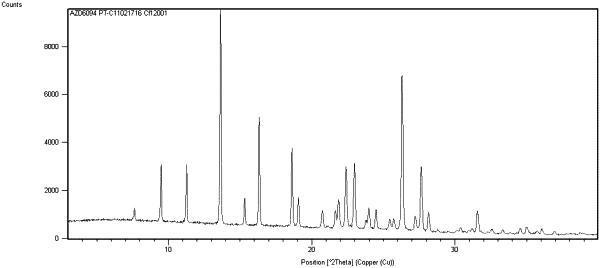
|
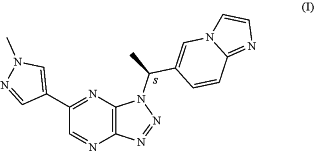
1. A process for the preparation of Savolinitib (I)
 comprising the preparation of (1S)-1-imidazo[1,2-a]pyridin-6-ylethanamine (III),
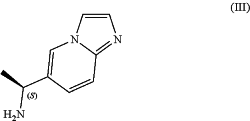 or a pharmaceutically acceptable salt thereof, comprising the steps of (i) asymmetric enzymatic transamination of 1-imidazo[1,2-a]pyridin-6-ylethanone (II),
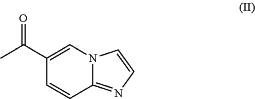 in the presence of an amine transaminase, pyridoxal phosphate, and an amine source; and (ii) isolation of (1S)-1-imidazo[1,2-a]pyridin-6-ylethanamine (III), or a pharmaceutically acceptable salt thereof.
|