| CPC C07D 401/14 (2013.01) [C07D 215/38 (2013.01); C07D 215/42 (2013.01); C07D 215/46 (2013.01); C07D 401/04 (2013.01); C07D 403/04 (2013.01); C07D 405/14 (2013.01); C07D 409/14 (2013.01); C07D 413/14 (2013.01); C07D 417/14 (2013.01); C07D 471/08 (2013.01); C07D 491/048 (2013.01); C07F 9/65031 (2013.01); C07F 9/6506 (2013.01)] | 23 Claims |
|
1. A compound, wherein the compound is of formula I*:
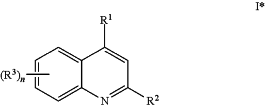 or a pharmaceutically acceptable salt thereof, wherein:
R1 is an optionally substituted imidazole, pyrazole, pyrrole, furan, or pyridine;
R2 is —NRR5,
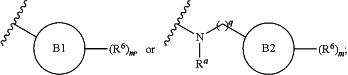 each R3 is independently halogen, —OMe, —OEt, —NR2, or —SR;
Ring B1 is substituted phenyl;
Ring B2 is a 4- to 7-membered saturated or partially unsaturated carbocyclic or heterocyclic ring having 1 to 3 heteroatoms independently selected from nitrogen, oxygen, and sulfur; or a 5- to 6-membered heteroaryl ring having 1 to 4 heteroatoms independently selected from nitrogen, oxygen, and sulfur;
each R is independently hydrogen or an optionally substituted group selected from C1-6 aliphatic; benzyl; phenyl; a 4- to 7-membered saturated or partially unsaturated carbocyclic or heterocyclic ring having 1 to 3 heteroatoms independently selected from nitrogen, oxygen, and sulfur; and a 5- to 6-membered heteroaryl ring having 1 to 4 heteroatoms independently selected from nitrogen, oxygen, and sulfur;
R5 is —(CH2)2-4OH, —(CH2)2-4O(CH2)1-5CO2H, —(CH2)2-4O(CH2)1-5CO2C1-4alkyl, —(CH2)0-3CH(CH2OH)2, —(CH2)2-4OC1-4alkyl, —(CR2)0-5CO2R, —(CR2)0-5CONR2, —(CR2)0-4C(O)NR(CR2)0-4CO2R, —(CR2)0-4C(O)NR(CR2)0-4CONR2, —(CR2)0-4NRC(O)R, —(CR2)0-4SO3R, —(CR2)0-4SO2NR2, —(CR2)0-4 OSO2NR2, —(CR2)0-4NRSO2R, —(CR2)0-4NRSO2OR, —(CR2)0-4OP(OR)2, —(CR2)0-4OP(O)(OR)2, —(CR2)0-4P(O)(OR)2, —(CR2)0-4OP(O)(H)OR;
each R6 is independently halogen, —CN, —COR, —(CR2)0-4CO2R, —(CR2)0-4CONR2, —OR, —(CR2)1-4OR, —NR2, —(CR2) 1-4NR2, —NRC(O)OR, —NRC(O)R, —NRC(O)NR2, —SR, —SO2R, —S(O)R, —(CR2)0-4SO3R, —(CR2)0-4SO2NR2, —(CR2)0-4OSO2NR2, —(CR2)0-4NRSO2R, —(CR2)0-4NRSO2OR, —(CR2)0-4OP(OR)2, —(CR2)0-4OP(O)(OR)2, —(CR2)0-4P(O)(OR)2, —(CR2)0-4OP(O)(H)OR, —B(OR)2, or RB,
RB and RC, independently, are an optionally substituted group selected from C1-6 aliphatic; phenyl; a 4- to 10-membered saturated or partially unsaturated monocyclic or bicyclic carbocyclic or heterocyclic ring having 1 to 3 heteroatoms independently selected from nitrogen, oxygen, and sulfur; and a 5- to 6-membered heteroaryl ring having 1 to 4 heteroatoms independently selected from nitrogen, oxygen, and sulfur;
each Ra is independently H or C1-6alkyl;
each m is 1, 2, 3, or 4;
n is 1, 2, 3, or 4;
q is 0, 1, or 2.
|