| CPC C07C 29/48 (2013.01) [B01J 23/26 (2013.01); B01J 35/617 (2024.01); B01J 35/618 (2024.01); B01J 35/633 (2024.01); B01J 35/635 (2024.01); C07C 29/09 (2013.01); C07C 29/74 (2013.01); C07C 2523/26 (2013.01)] | 28 Claims |
|
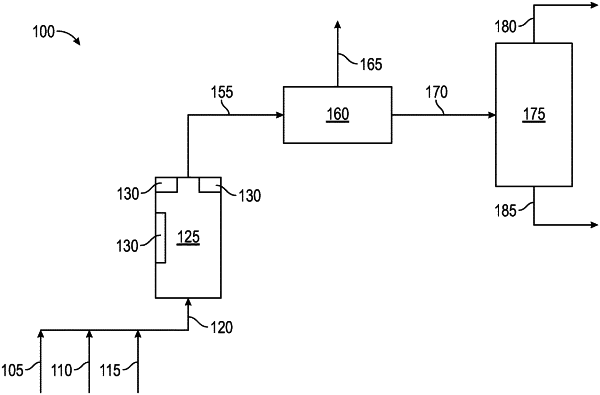
1. A process for converting methane into methanol, the process comprising:
(i) contacting methane, water, and a supported chromium catalyst comprising chromium in a hexavalent oxidation state with a light beam at a wavelength in the UV-visible spectrum in an oxidizing atmosphere in a single reactor to form a reaction product comprising methanol, wherein:
the oxidizing atmosphere comprises air; and
a molar ratio of molecular oxygen in the oxidizing atmosphere to chromium of the supported chromium catalyst is at least 2:1;
(ii) discharging a reactor effluent containing the reaction product from the single reactor; and
(iii) separating methanol from the reaction product.
|