| CPC A61M 5/14232 (2013.01) | 12 Claims |
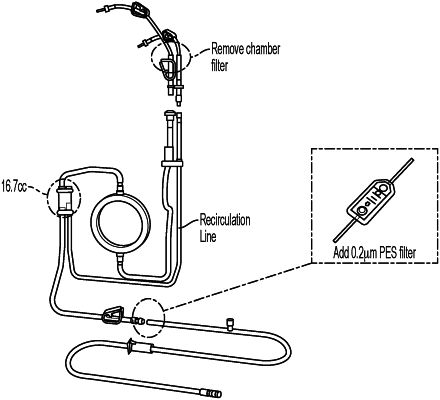
|
1. A kit comprising a volume of solution to be administered to a patient and an infusion device for administering the volume of solution to the patient by intravenous infusion,
wherein the volume of solution comprises one or more antibodies selected from the group consisting of: (i) one or more monoclonal and/or polyclonal antibodies (mAbs and/or pAbs), (ii) one or more antibody drug conjugates (ADCs), (iii) one or more lipid drug conjugates (LDCs), and (iv) intravenous immunoglobulin (IVIg);
wherein the infusion device comprises (a) a pump, and (b) a disposable infusion set comprising a tubing line or lines fluidly connecting an intravenous (IV) bag or other receptacle containing the volume of solution to the pump, and/or the pump to the patient, and
wherein the pump administers the volume of solution at a dosing rate of at least 35 mg of the one or more antibodies per minute and at a total antibody concentration of less than or equal to 20 mg/mL.
|