| CPC A61K 39/104 (2013.01) [A61K 31/738 (2013.01); C07H 1/00 (2013.01); C07H 3/06 (2013.01); A61K 39/00 (2013.01)] | 4 Claims |
|
1. A method of synthesizing an O-antigen trisaccharide of a Pseudomonas aeruginosa serotype O11, comprising:
(a) synthesizing a D-glucose building block of formula II,
(b) synthesizing an L-fucosamine building block of formula III,
(c) synthesizing a D-fucosamine building block of formula IV by:
(i) reacting 3,4,6-tri-O-acetyl-D-glucal with azidotrimethylsilane (TMS-N3) and diphenyl disenenide (Ph2Se2) with iodobenzene diacetate (PhI(OAc)2) to obtain 1-selenophenyl-2 azido glucose,
(ii) removing acetyl under alkaline conditions, and
(iii) methylating 6-C by incubation with 4-toluenesulfonyl chloride, to obtain D-fucosamine,
(d) synthesizing a disaccharide fragment by attaching the D-glucose building block to the L-fucosamine building block, wherein a chemical structural formula of the disaccharide fragment is as shown in formula V:
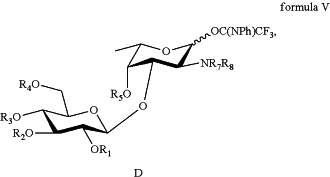 and
(e) linking together the disaccharide fragment and the D-fucosamine building block;
wherein:
the D-glucose building block or the L-fucosamine building block is linked with the D-fucosamine building block through a 1,2-α-cis-glycosidic bond,
the D-glucose building block is linked with the L-fucosamine building block through a 1,2-α-trans-glycosidic bond, and
construction of the 1,2-α-cis-glycosidic bond is conducted in a mixed solvent; and
the mixed solvent comprises two or more of dichloromethane, diethyl ether, and thiophene,
formula II, formula III, and formula IV have the following structures:
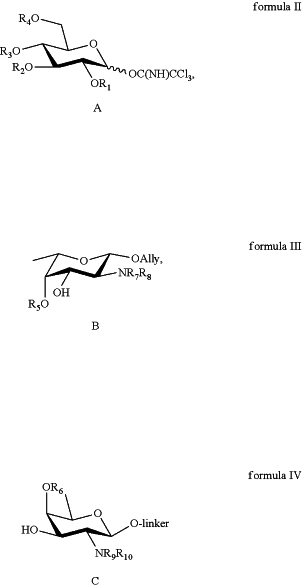 the linker comprises —(CH2)n—N—Y1Y2, or O—(CH2)n—SY1(Y2), or O—(CH2)n—N3, where:
n=1 to 10;
Y1 is hydrogen (H) or benzyl (Bn);
Y2 is hydrogen (H) or benzyl methoxycarbonyl (Cbz);
R1 comprises hydrogen (H), an ester group, acetyl (Ac), benzoyl (Bz), pivaloyl (Piv), chloracetyl (ClAc), levulinyl (Lev) and allyl carbonyl (Alloc);
R2, R3, and R4 are hydrogen (H) or an ester group and an ether group, and comprise acetyl (Ac), benzoyl (Bz), pivaloyl (Piv), chloracetyl (ClAc), levulinyl (Lev), allyl carbonyl (Alloc), benzyl (Bn), p-methoxybenzyl (pMBn), allyl (All), triphenylmethyl (Tr), monomethoxy triphenyl methyl (Mmt) and a silyl ether group;
R5 and R6 comprise hydrogen (H), an ether group, benzyl (Bn), p-methoxybenzyl (pMBn), allyl (All), triphenylmethyl (Tr), monomethoxy triphenyl methyl (Mmt) and a silyl ether group; and
R7, R8, R9, and R10 comprise hydrogen (H), nitrogen (N) or acetyl (Ac).
|