| CPC A61K 38/27 (2013.01) [A61K 9/08 (2013.01); A61K 47/12 (2013.01); A61K 47/20 (2013.01); A61K 47/26 (2013.01); A61K 47/60 (2017.08); C08G 65/3348 (2013.01); C08G 2650/06 (2013.01)] | 21 Claims |
|
1. A container comprising a polymeric human growth hormone (hGH) prodrug or a pharmaceutically acceptable salt thereof of formula (Ia) or (Ib)
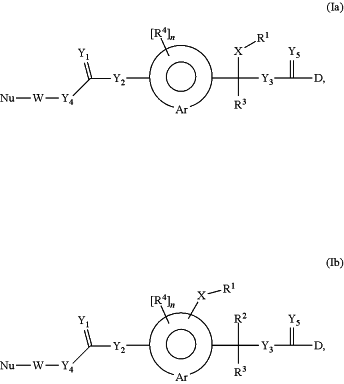 wherein
—D is a hGH moiety connected to the rest of the molecule through an amine functional group;
n is 0, 1, 2, 3, or 4;
—X— is a chemical bond or a spacer;
═Y1 is selected from the group consisting of ═O and ═S;
—Y2— is selected from the group consisting of —O— and —S—;
—Y3— is selected from the group consisting of —O— and —S—;
—Y4— is selected from the group consisting of —O—, —NR5— and —C(R6R6a)—;
═Y5 is selected from the group consisting of ═O and ═S;
—R1 comprises a moiety of formula (IIc):
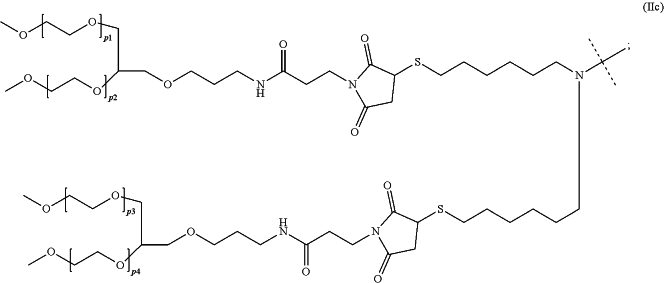 wherein:
p1, p2, p3, and p4 are independently an integer ranging from 180 to 240;
—R2, —R3, —R5, —R6 —R6a are independently of each other selected from the group consisting of —H, methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl, sec-butyl, tert-butyl, n-pentyl, 2-methylbutyl, 2,2-dimethylpropyl, n-hexyl, 2-methylpentyl, 3-methylpentyl, 2,2-dimethylbutyl, 2,3-dimethylbutyl and 3,3-dimethylpropyl;
—R4 is selected from the group consisting of methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl, sec-butyl, tert-butyl, n-pentyl, 2-methylbutyl, 2,2-dimethylpropyl, n-hexyl, 2-methylpentyl, 3-methylpentyl, 2,2-dimethylbutyl, 2,3-dimethylbutyl and 3,3-dimethylpropyl;
—W— is selected from the group consisting of C1-20 alkyl optionally interrupted by one or more groups selected from the group consisting of C3-10 cycloalkyl, 8- to 30-membered carbopolycyclyl, 3- to 10-membered heterocyclyl, —C(O)—, —C(O)N(R7)—, —O—, S— and —N(R7)—;
—Nu is a nucleophile selected from the of group consisting of —N(R7R7a), —N(R7OH), —N(R7)—N(R7aR7b), —S(R7), —COOH,
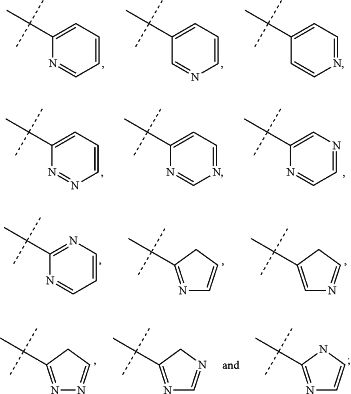 —Ar— is selected from the group consisting of
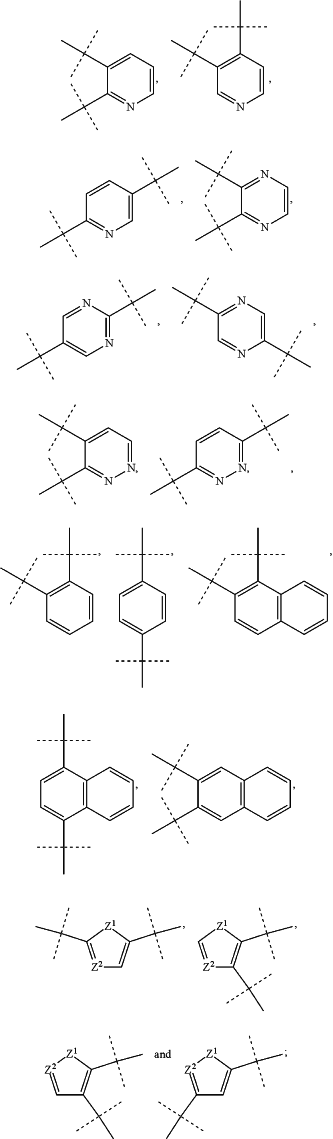 wherein
dashed lines indicate attachment to the rest of the prodrug,
—Z1— is selected from the group consisting of —O—, —S— and —N(R7)—, and
—Z2 is —N(R7)—; and
—R7 —R7a, —R7b are independently of each other selected from the group consisting of —H, C1-6 alkyl, C2-6 alkenyl and C2-6 alkynyl;
wherein the prodrug of formula (Ia) and (Ib) is optionally further substituted.
|