| CPC A61B 17/221 (2013.01) [A61B 17/320725 (2013.01); A61B 2017/00867 (2013.01); A61B 2017/22079 (2013.01); A61B 2090/3966 (2016.02)] | 20 Claims |
|
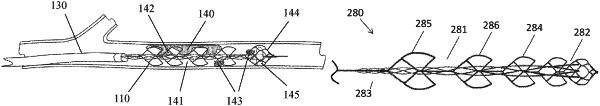
1. A clot retrieval device for removing occlusive clot from a blood vessel, the device comprising:
an inner elongate body comprising a collapsed delivery configuration and an expanded deployed configuration; and
an outer elongate body at least partially overlying the inner elongate body, the outer elongate body being expandable to a radial extent which is greater than the radial extent of the inner elongate body in the deployed configuration to define a clot reception space;
wherein the outer elongate body comprises a plurality of clot inlet openings and a plurality of clot engaging regions, wherein the clot engaging regions are adapted, on engagement with the occlusive clot, to urge the occlusive clot towards the clot inlet openings and into the clot reception space between the outer elongate body and the inner elongate body;
wherein a diameter of the inner elongate body varies along the length of the inner elongate body and a diameter of the outer elongate body varies along the length of the outer elongate body;
wherein the radial force profile of the device varies along the length of the device; and
wherein a size of a first clot inlet opening of the plurality of clot inlet openings and a second clot inlet opening of the plurality of clot inlet openings is different than a size of the second clot inlet opening of the plurality of clot inlet openings and a third clot inlet opening of the plurality of clot inlet openings.
|