| CPC C25B 3/27 (2021.01) [C07C 231/12 (2013.01); C07C 237/46 (2013.01); C25B 3/07 (2021.01); C25B 3/09 (2021.01); C25B 3/11 (2021.01); C25B 15/081 (2021.01); C25B 15/083 (2021.01); C25B 15/087 (2021.01)] | 15 Claims |
|
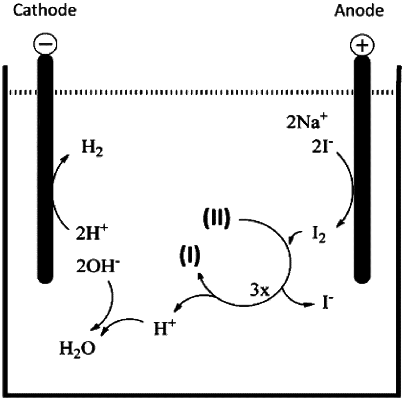
1. A process for the preparation of N,N′-bis-(2,3-dihydroxypropyl)-5-hydroxy-2,4,6-triiodo-1,3-benzenedicarboxamide (I), comprising the steps of:
a) dissolving N,N′-(2,3-dihydroxypropyl)-5-hydroxy-1,3-benzenedicarboxamide (II) in a reaction medium in the presence of molecular iodine (I2) and
b) iodinating said compound (II) with the molecular iodine (I2) to obtain said compound (I), wherein the reaction medium is an aqueous solution and the molecular iodine (I2) is in situ electrochemically generated from a source of iodide ions (I−) and wherein steps a) and b) are carried out in
galvanostatic mode using an undivided electrolytic cell or
in potentiostatic mode using an electrolytic cell formed by two-compartments divided by a porous septum or by a ionic exchange membrane.
|