| CPC C07J 9/00 (2013.01) [A61K 9/0019 (2013.01); A61K 9/0095 (2013.01); A61K 9/2063 (2013.01); A61K 9/4866 (2013.01); A61K 31/575 (2013.01); C07J 9/005 (2013.01); C07J 13/005 (2013.01); C07J 13/007 (2013.01); C07J 31/006 (2013.01)] | 20 Claims |
|
1. A compound of Formula (I):
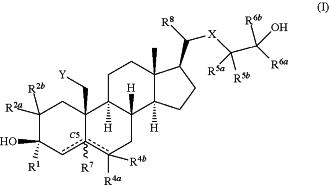 or a pharmaceutically acceptable salt thereof;
wherein:
R1 is hydrogen, C1-C6 alkyl, C2-C6 alkenyl, C2-C6 alkynyl, carbocyclyl, or heterocyclyl;
each of R2a and R2b is independently hydrogen, C1-C6 alkyl, halo, cyano,—ORA, or —NRBRC, or
R2a and R2b together with the carbon atom to which they are attached form a 3-7 membered ring;
each of R4a and R4b is independently absent, hydrogen, C1-C6 alkyl, or halo;
X is —C(RX)2— or —O—, wherein RX is independently hydrogen, halo, or one RX group and R5b are joined to form a double bond;
Y is —ORY, wherein RY is hydrogen, C1-C6 alkyl, C2-C6 alkenyl, C2-C6 alkynyl, carbocyclyl, heterocyclyl, aryl, heteroaryl, —C(O)RA, —C(O)ORA, —C(O)NRBRC, or —S(O)2RD;
each instance of R5a and R5b is independently hydrogen, halo, or C1-C6 alkyl;
each of R6a and R6b is independently hydrogen, C1-C6 alkyl, C2-C6 alkenyl, C2-C6 alkynyl, carbocyclyl, heterocyclyl, aryl, or heteroaryl, or
R6a and R6b, taken together with the carbon atom to which they are attached, form a 3-6 membered ring; or
R5a and R6a, together with the carbon atoms to which they are attached, form a 3-6-membered ring; and
R7 is absent or hydrogen in the alpha configuration;
R8 is hydrogen, halo, C1-6alkyl, carbocyclyl, or —ORA;
 represents a single or double bond, wherein when one represents a single or double bond, wherein when one  is a double bond, the other is a double bond, the other  is a single bond; is a single bond;wherein when the
 between —CR7 and —CR4aR4b is a double bond, then one of R4a or R4b is absent; and between —CR7 and —CR4aR4b is a double bond, then one of R4a or R4b is absent; andwhen one of the
 is a double bond, R7 is absent; is a double bond, R7 is absent;RA is hydrogen, C1-C6 alkyl, carbocyclyl, heterocyclyl, aryl, or heteroaryl;
each of RB and RC is independently hydrogen, C1-C6 alkyl, carbocyclyl, heterocyclyl, aryl, heteroaryl, or taken together with the atom to which they are attached form a 3-7-membered ring; and
RD is hydrogen, C1-C6 alkyl, carbocyclyl, heterocyclyl, aryl, or heteroaryl.
|