| CPC C07D 471/04 (2013.01) [C07D 401/12 (2013.01); C07D 401/14 (2013.01); C07D 403/12 (2013.01); C07D 403/14 (2013.01); C07D 405/12 (2013.01); C07D 471/18 (2013.01); C07D 487/04 (2013.01); C07D 487/08 (2013.01); C07D 491/04 (2013.01); C07D 495/04 (2013.01); C07D 498/08 (2013.01); C07D 498/18 (2013.01)] | 22 Claims |
|
1. A compound having the structural formula (III):
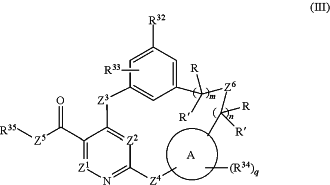 or a pharmaceutically acceptable form or an isotope derivative thereof,
wherein
Ring A is a 6-membered aryl or heteroaryl;
X1 is NH;
Z1 is CH or N,
Z2 is CH, CF or N,
each of Z3 and Z4 is NH;
Z6 is NH, NCH3, CH2 or O;
R32 is a 5- or 6-membered heteroaryl group, each substituted with 0-3 R32a;
R32a is independently at each occurrence, H, OCF3, CN, —(CH2)rORb, —(CH2)r SRb, —(CH2)rC(O)Rb, —(CH2)rC(O)ORb, —(CH2)rOC(O)Rb, (CH2)rNRgRg, —(CH2)rC(O)NRgRg, —(CH2)rNRbC(O)Rc, —(CH2)rNRbC(O)ORc, —NRbC(O)NRgRg, —S(O)vNRgRg, —NRbS(O)vRc, —S(O)vRc, C1-6 alkyl substituted with 0-3 Ra, C1-6 haloalkyl, C2-6 alkenyl substituted with 0-3 Ra, 3- to 6-membered cycloalkyl substituted with 0-3 Ra, or 3- to 6-membered heterocycloalkyl comprising 1-3 heteroatoms selected from N, O and S substituted with 0-3 Ra;
each of R33 and R34 is independently selected from H, F, Cl, CN, ORg, CH3, CD3, CF3, OCD3, OCF3 and —(CH2)p-Q;
R35 is H, F, a C1-C3 alkyl and CD3, provided that R35 is not F when X1 is O or N;
Ra at each occurrence is independently H, F, Cl, Br, OCF3, CF3, CHF2, CN, —(CH2)rORb, —(CH2)rSRb, —(CH2)rC(O)Rb, —(CH2)rC(O)ORb, —(CH2)rOC(O)Rb, —(CH2)rNRgRg, —(CH2)rC(O)NRgRg, —(CH2)rNRbC(O)Rc, —(CH2)rNRbC(O)ORc, —NRbC(O)NRgRg, —S(O)vNRgRg, —NRbS(O)vRc, —S(O)Rc, —S(O)2Rc, C1-6 alkyl substituted with 0-3 Rf, C1-6 haloalkyl, 3- to 6-membered cycloalkyl substituted with 0-3 Rf, or 3- to 6-membered heterocycloalkyl comprising 1-3 heteroatoms selected from N, O and S substituted with 0-3 Rf;
Rb is H, C1-6 alkyl substituted with 0-3 Rd, C1-6 haloalkyl, C3-6 cycloalkyl substituted with 0-2 Rd, or 5- to 7-membered heterocycloalkyl comprising 1-3 heteroatoms selected from N, O and S substituted with 0-3 Rf or (CH2)r-phenyl substituted with 0-3 Rd;
Rc is C1-6 alkyl substituted with 0-3 Rf, (CH2)r—C3-6 cycloalkyl substituted with 0-3 Rf or (CH2)r-phenyl substituted with 0-3 Rf,
Rd is independently at each occurrence, hydrogen, F, Cl, Br, OCF3, CF3, CN, NO2, —ORe, —(CH2)rC(O)Rc, —NReRe, —NReC(O)ORc, C1-6 alkyl or (CH2)r-phenyl substituted with 0-3 Rf;
Re is independently at each occurrence, hydrogen, C1-6 alkyl, C3-6 cycloalkyl or (CH2)r-phenyl substituted with 0-3 Rf;
Rf is independently at each occurrence, hydrogen, halo, CN, NH2, OH, C3-6 cycloalkyl, CF3, O(C1-6 alkyl) or a 5- to 7-membered heterocycloalkyl comprising 1-3 heteroatoms selected from N, O and S;
Rg at each occurrence is independently H, C1-4 alkyl substituted with 0-3 Rf, CF3, C3-10 cycloalkyl substituted with 0-1 Rf, (CH)r-phenyl substituted with 0-3 Rd or 5- to 7-membered heterocycloalkyl comprising 1-3 heteroatoms selected from N, O and S substituted with 0-3 Rd;
Q is a water solubilizing group, optionally selected from OH, OR, NRR′, heterocyclic and heteroaryl groups, wherein R and R′, together with the nitrogen atom to which they are bound, form a 4- to 7-membered ring comprising 0-2 heteroatoms selected from O, NR, S and SO2;
R is H;
R′ is H,
m is 1;
n is 1;
p is 0, 1, 2, 3 or 4;
q is 0, 1, 2, 3 or 4;
v is 0, 1, or 2; and
r is 0, 1, 2, 3, 4 or 5.
|