| CPC C07D 401/12 (2013.01) [A61P 25/18 (2018.01); C07D 401/14 (2013.01); C07D 405/14 (2013.01); C07D 409/14 (2013.01); C07D 413/14 (2013.01)] | 19 Claims |
|
1. A method of inhibiting a DAAO, comprising contacting a cell with a compound of formula (I),
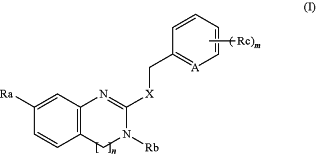 wherein n is 0 or 1,
X is —S—, —S(═O)— or —NRn—; wherein
Rn is H or
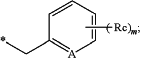 A is —CH, —CRc or N;
Ra is —C(═O)ORa1, —ORa2, —O—C(═O)Ra3 or —O—C(═O)-T-ORa4; wherein
Ra1 is H or linear or branched C1-15alkyl;
Ra2 is H, linear or branched C1-15alkyl, phosphonate, diarylphosphonate or an O-protecting group;
Ra3 and Ra4 are independently a protecting group, linear or branched C1-15alkyl, linear or branched C2-15alkenyl, -T-C3-10cycloalkyl, -T-NHRa3p, -T-C3-10cycloalkenyl, -T-C6-10aryl, -T-C5-10heteroaryl, -T-NH—C(═O)—O—C1-10alkyl, -T-adamantyl or —C1-3alkylene-C6-10aryl where the alkylene is substituted with -T-NHRa3p;
Ra3p is H or an N-protecting group;
Rb is H, linear or branched C1-15alkyl, linear or branched C2-15alkenyl, C1-3alkoxy-C1-15alkyl-, -T′-C3-10cycloalkyl, -T′-C3-10cycloalkenyl, -T′-C6-10 aryl or -T′-C5-10heteroaryl;
Rc each is independently linear or branched C1-15alkyl, linear or branched C1-15alkoxyl, unprotected or protected hydroxyl group, or —C1-10alkylene-Y—C6-10heteroaryl wherein —Y— is —CH2—, —NH—, —O— or —S—;
symbol * represents the bonding position;
m is an integer from 0 to 4;
-T- is absent, C1-3alkylene or C2-3alkenylene;
-T′- is C1-3alkylene or C2-3alkenylene; and
wherein the heteroaryl contains at least one heteroatom, each heteroatom being independently S, N or O;
wherein the alkyl, alkenyl, alkoxy, cycloalkyl, aryl, heteroaryl, alkylene and alkenylene are each independently unsubstituted or substituted with at least one substituent;
wherein the substituent is each independently a halogen, a protecting group, protected or unprotected amino group, nitro, nitroso, linear or branched C1-15 alkyl, or linear or branched C1-15 alkoxy or C3-10cycloalkyl; and
when Rb is H, the tautomers are included,
with the proviso that
when X is —S— or —S(═O)—, Ra is —ORa2 and Ra2 is H or linear or branched C1-15alkyl, then A is —CH or —CRc;
when X is —S— or —S(═O)— and Ra is —C(═O)ORa1, Rb is linear or branched C6-15alkyl, linear or branched C6-15alkenyl, C1-3alkoxy-C1-15alkyl-, -T′-C3-10cycloalkyl, -T′-C3-10cycloalkenyl, -T′-C6-10 aryl or -T′-C5-10heteroaryl;
or a pharmaceutically acceptable salt thereof.
|