| CPC C07D 239/48 (2013.01) [A61K 31/505 (2013.01); C07D 239/38 (2013.01); C07D 239/47 (2013.01); C07D 401/04 (2013.01); C07D 401/12 (2013.01); C07D 403/12 (2013.01); C07D 405/12 (2013.01); C07D 409/12 (2013.01)] | 6 Claims |
|
1. A method for preparing a compound of formula (IIb):
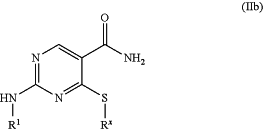 the method comprising contacting a compound of formula (IIc):
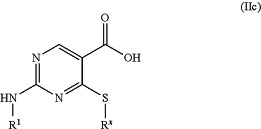 with NH4Cl in the presence of a coupling agent and a base, in a solvent, wherein:
R1 is substituted or unsubstituted C1-8 alkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted non-aromatic heterocyclyl, substituted or unsubstituted alkyl-(saturated or partially saturated cycloalkyl), or substituted or unsubstituted alkylheterocyclyl, provided that R1 is not 1-aminocyclohexyl;
Rx is a C1-2 alkyl; and
wherein when a C1-8 alky group is substituted, the C1-8 alkyl group is substituted with halogen, alkyl, hydroxyl, alkoxy, alkoxyalkyl, amino, alkylamino, carboxy, nitro, cyano, thiol, thioether, imine, imide, amidine, guanidine, enamine, aminocarbonyl, acylamino, phosphonato, phosphine, thiocarbonyl, sulfonyl, sulfone, sulfonamide, ketone, aldehyde, ester, urea, urethane, oxime, hydroxyl amine, alkoxyamine, aralkoxyamine, N-oxide, hydrazine, hydrazide, hydrazone, azide, isocyanate, isothiocyanate, cyanate, thiocyanate, B(OH)2, or O(alkyl)aminocarbonyl,
wherein when a group, other than a C1-8 alkyl group, is substituted, the group is substituted with halogen, alkyl, hydroxyl, alkoxy, alkoxyalkyl, amino, alkylamino, carboxy, nitro, cyano, thiol, thioether, imine, imide, amidine, guanidine, enamine, aminocarbonyl, acylamino, phosphonato, phosphine, thiocarbonyl, sulfonyl, sulfone, sulfonamide, ketone, aldehyde, ester, urea, urethane, oxime, hydroxyl amine, alkoxyamine, aralkoxyamine, N-oxide, hydrazine, hydrazide, hydrazone, azide, isocyanate, isothiocyanate, cyanate, thiocyanate, oxo, B(OH)2, O(alkyl)aminocarbonyl, cycloalkyl, heterocyclyl, aryl, heteroaryl, aryloxy, aralkyloxy, heterocyclyloxy or heterocyclylalkoxy, and
wherein the coupling agent is selected from the group consisting of HATU, CDI, HBTU, and ethyl chloroformate, and the base is selected from the group consisting of DIEA, TEA, and potassium carbonate.
|