| CPC C01B 3/16 (2013.01) [B01J 21/02 (2013.01); B01J 23/80 (2013.01); B01J 35/19 (2024.01); B01J 37/0236 (2013.01); B01J 37/031 (2013.01); B01J 37/06 (2013.01); B01J 37/08 (2013.01); B01J 37/18 (2013.01); C01B 2203/0283 (2013.01); C01B 2203/1076 (2013.01); C01B 2203/1088 (2013.01)] | 16 Claims |
|
1. A method of preparing hydrogen, comprising providing a catalyst for a water gas shift reaction, to perform a water gas shift reaction,
wherein the catalyst for the water gas shift reaction comprises a catalytically active component containing 40 to 80 mol % of copper (Cu), 15 to 50 mol % of zinc (Zn), and 1 to 13 mol % of aluminum (Al), relative to all metals of the catalyst,
wherein an aluminum-rich layer is present in a surface layer of a particle of the catalyst,
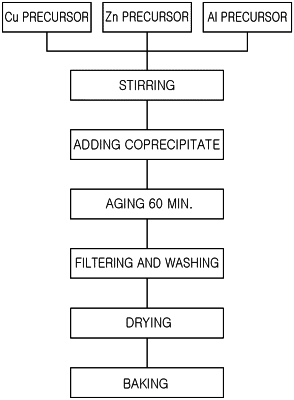
wherein the catalyst is prepared by a catalyst preparation method comprising a copper-zinc coprecipitation operation of injecting a metal precursor solution containing a Cu precursor and a Zn precursor into a precipitant solution to coprecipitate copper and zinc, to produce a copper-zinc coprecipitate;
an Al precipitation operation of injecting an Al precursor solution into a solution containing the copper-zinc coprecipitate to precipitate aluminum on a surface of the copper-zinc coprecipitate, to prepare a CuZnAl catalyst precursor material having an aluminum-rich layer on a surface; and
a baking operation of baking the CuZnAl catalyst precursor material to prepare a CuZnAl catalyst,
wherein the copper-zinc coprecipitation operation is performed by aging a mixture of the metal precursor solution containing the Cu precursor and the Zn precursor, and the precipitant solution within a temperature range of room temperature (20° C.) to 80° C. for 30 to 180 minutes,
wherein the Al precursor solution is injected, after a phenomenon after which a pH is reduced to a range of 0.05 to 0.2 and is then returned during the aging.
|