| CPC A61K 47/549 (2017.08) [A61K 47/62 (2017.08); C07H 5/06 (2013.01); C07H 7/02 (2013.01); C07H 9/02 (2013.01); C07H 9/04 (2013.01); C07H 15/203 (2013.01); C07H 17/00 (2013.01); C07H 17/02 (2013.01); C07H 19/02 (2013.01); C07H 19/044 (2013.01); H03L 7/0814 (2013.01); H03L 7/0818 (2013.01); H03L 7/0998 (2013.01)] | 5 Claims |
|
1. A compound of the formula:
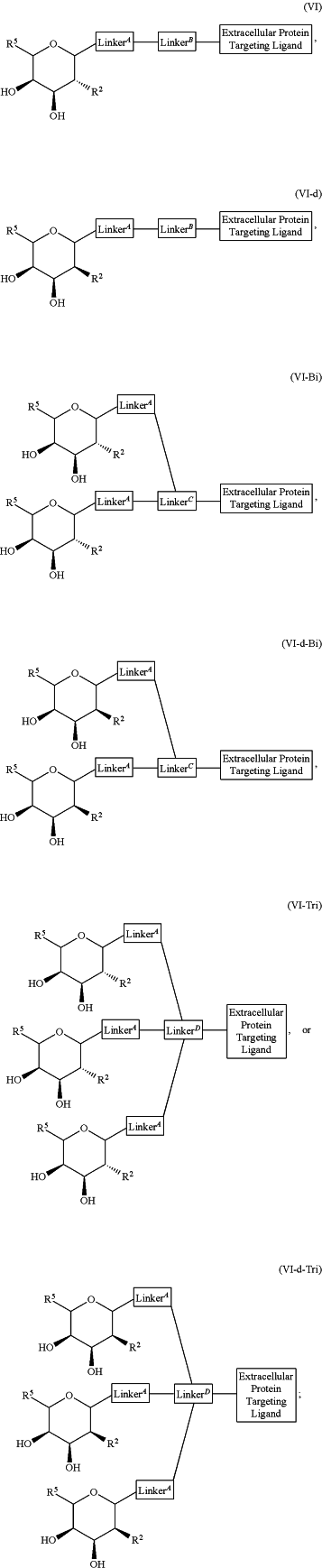 or a pharmaceutically acceptable salt thereof;
wherein:
Extracellular Protein Targeting Ligand is an IgG binding ligand selected from the group consisting of Fc-III, FcBP-1, FcBP-2, and Fc-III-4c;
R2 is selected from the group consisting of:
(i) aryl, heterocycle, and heteroaryl containing 1 or 2 heteroatoms independently selected from N, O, and S, each of which aryl, heterocycle, and heteroaryl is optionally substituted with 1, 2, 3, or 4 substituents;
(ii)
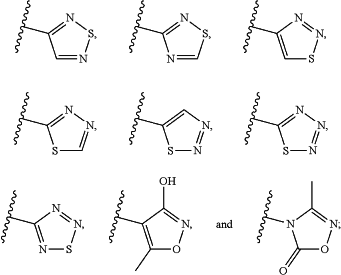 (iii)-NR8—S(═O)—R3, —NR8—C(═S)—R3, —NR8—S(═O)(NR6)—R3, —N═S(═O)(R3)2, —NR8C(═O)NR9S(═O)2R3, —NR8—S(═O)2—R10, and —NR8—C(NR6)—R3 each of which is optionally substituted with 1, 2, 3, or 4 substituents; and
(iv) hydrogen, R10, alkyl-C(═O)—R3, —C(═O)—R3, alkyl, haloalkyl, —OC(═O)R3, and —NR8—C(═O)R10;
R10 is selected from the group consisting of aryl, alkyl-NR8—C(═O)—R3, alkyl-aryl, alkyl-heteroaryl with 1, 2, or 4 heteroatoms, alkyl-cyano, alkyl-OR6, alkyl-NR6R8, NR8—NR6—C(═O)R3, NR8—S(═O)2—R3, alkenyl, allyl, alkynyl, —NR6-alkenyl, —O-alkenyl, —NR6-alkynyl, —O-alkynyl, —NR6-heteroaryl, —NR6-aryl, —O-heteroaryl, and —O-aryl, each of which R10 is optionally substituted with 1, 2, 3, or 4 substituents;
R5 at each occurrence is independently selected from the group consisting of hydrogen, heteroalkyl, C0-C6alkyl-cyano, alkyl, alkenyl, alkynyl, haloalkyl, F, Cl, Br, I, aryl, arylalkyl, heteroaryl, heteroarylalkyl, heterocycle, heterocycloalkyl, haloalkoxy, —O-alkenyl, —O-alkynyl, C0-C6alkyl-OR6, C0-C6alkyl-SR6, C0-C6alkyl-NR6R7, C0-C6alkyl-C(═O)R3, C0-C6alkyl-S(═O)R3, C0-C6alkyl-C(═S)R3, C0-C6alkyl-S(═O)2R3, C0-C6alkyl-N(R8)—C(═O)R3, C0-C6alkyl-N(R8)—S(═O)R3, C0-C6alkyl-N(R8)—C(═S)R3, C0-C6alkyl-N(R8)—S(═O)2R3 C0-C6alkyl-O—C(═O)R3, C0-C6alkyl-O—S(═O)R3, C0-C6alkyl-O—C(═S)R3, —N═S(═O)(R3)2, C0-C6alkylN3, and C0-C6alkyl-O—S(═O)2R3, each of which is optionally substituted with 1, 2, 3, or 4 substituents;
R3 at each occurrence is independently selected from the group consisting of hydrogen, alkyl, heteroalkyl, haloalkyl, arylalkyl, heteroarylalkyl, alkenyl, alkynyl, aryl, heteroaryl, heterocycle, —OR8, and —NR8R9;
R6 and R7 are independently selected at each occurrence from the group consisting of hydrogen, heteroalkyl, alkyl, arylalkyl, heteroarylalkyl, alkenyl, alkynyl, aryl, haloalkyl, heteroaryl, heterocycle, -alkyl-OR8, -alkyl-NR8R9, C(═O)R3, S(═O)R3, C(═S)R3, and S(═O)2R3;
R8 and R9 are independently selected at each occurrence from the group consisting of hydrogen, heteroalkyl, alkyl, arylalkyl, heteroarylalkyl, alkenyl, alkynyl, aryl, heteroaryl, and heterocycle;
R21 is independently at each occurrence selected from the group consisting of hydrogen, alkyl, alkenyl, alkynyl, F, Cl, Br, I, hydroxyl, alkoxy, azide, amino, cyano, —NR6R7, —NR8S(═O)2R3, —NR8S(═O)R3, haloalkyl, heteroalkyl, aryl, heteroaryl, and heterocycle;
tt is independently selected from 2 or 3;
ss is 3-tt;
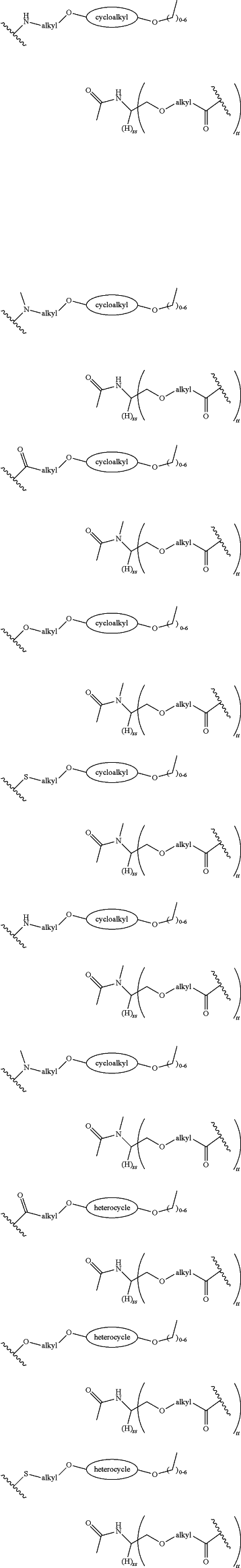
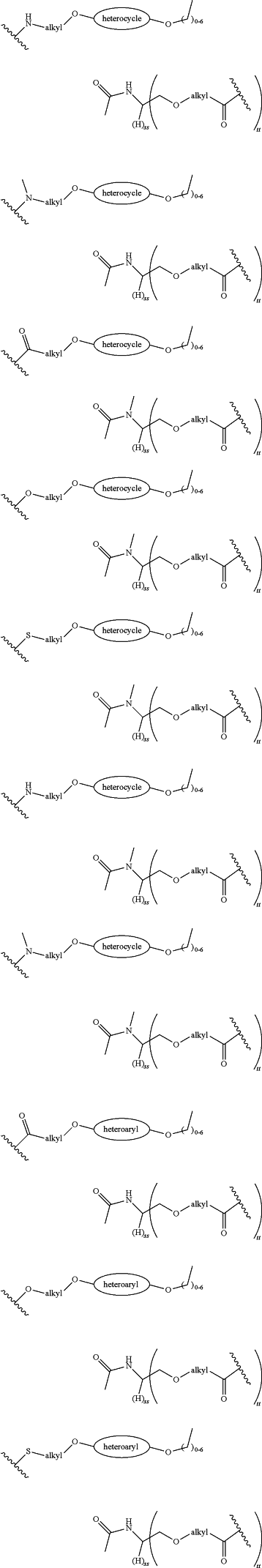
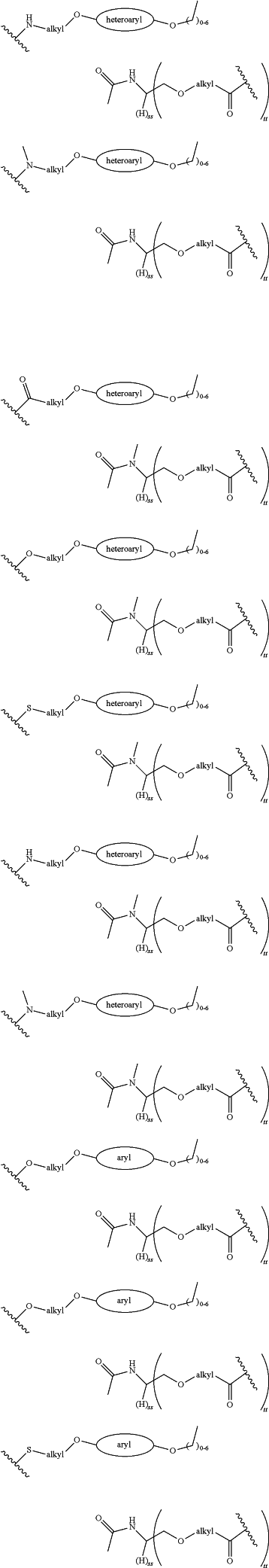
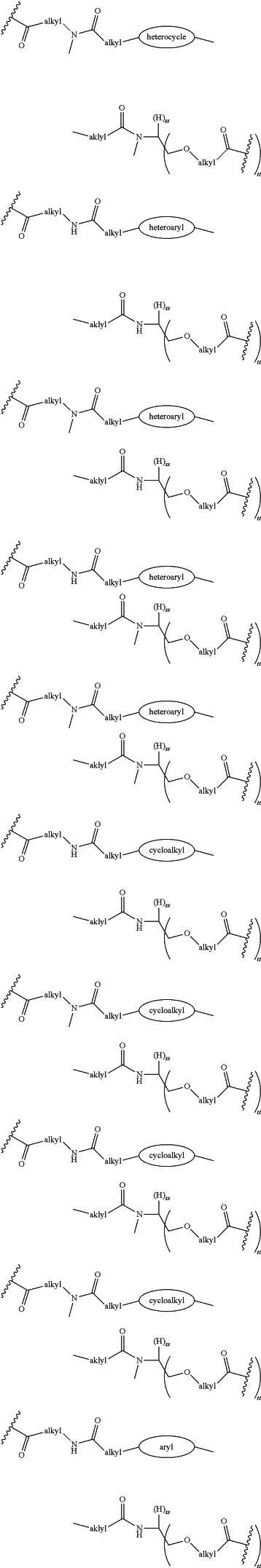
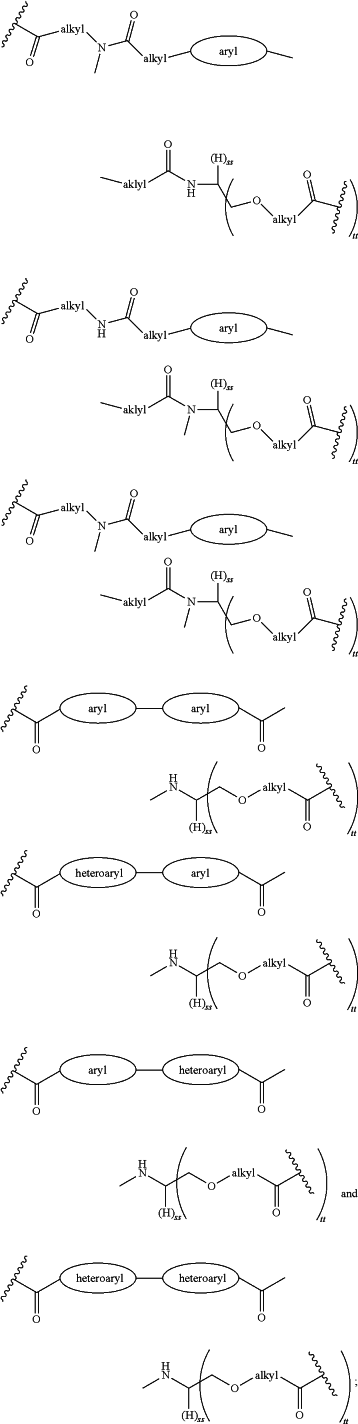
each LinkerA and LinkerB is independently selected from
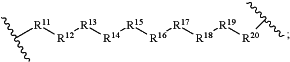 wherein:
R11, R12, R13, R14, R15, R16, R17, R18, R19, and R20 are independently at each occurrence selected from the group consisting of a bond, alkyl, —C(═O)—, —C(═O)O—, —OC(═O)—, —SO2—, —S(═O)—, —C(═S)—, —C(═O)NR6—, —NR6C(═O)—, —O—, —S—, —NR6—, —C(R21R21)—, —P(═O)(R3)O—, —P(═O)(R3)—, a divalent residue of a natural or unnatural amino acid, alkenyl, alkynyl, haloalkyl, alkoxy, aryl, heterocycle, heteroaryl, —CH2CH2—[O—(CH2)2]n—O—, —CH2CH2—[O—(CH2)2]n—NR6—, —CH2CH2—[O—(CH2)2]n—, —[—(CH2)2—O—]n—, —[O—(CH2)2]n—, —[O—CH(CH3)C(═O)]n—, —[C(═O)—CH(CH3)—O]n—, —[O—CH2C(═O)]n—, —[C(═O)—CH2—O]n—, a divalent residue of a fatty acid, and a divalent residue of an unsaturated or saturated mono- or di-carboxylic acid, each of which is optionally substituted with 1, 2, 3, or 4 substituents independently selected from R21;
n is independently selected at each instance from 0, 1, 2, 3, 4, 5, 6, 7, 8, 9, or 10;
LinkerC and LinkerD are each independently selected from the group consisting of:
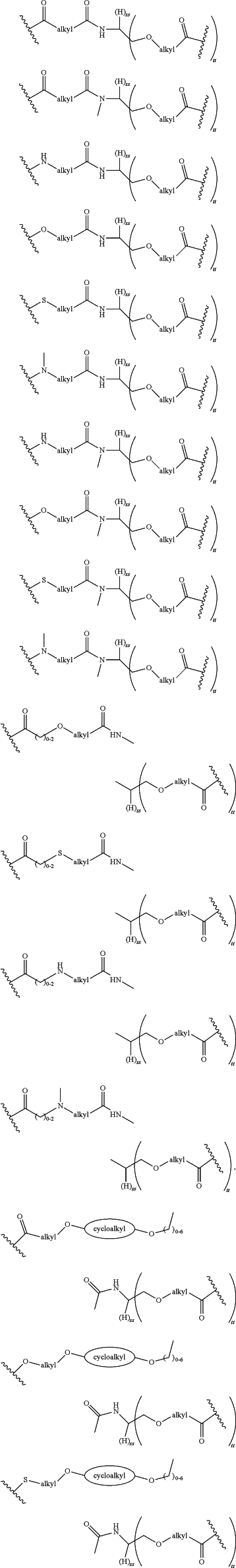    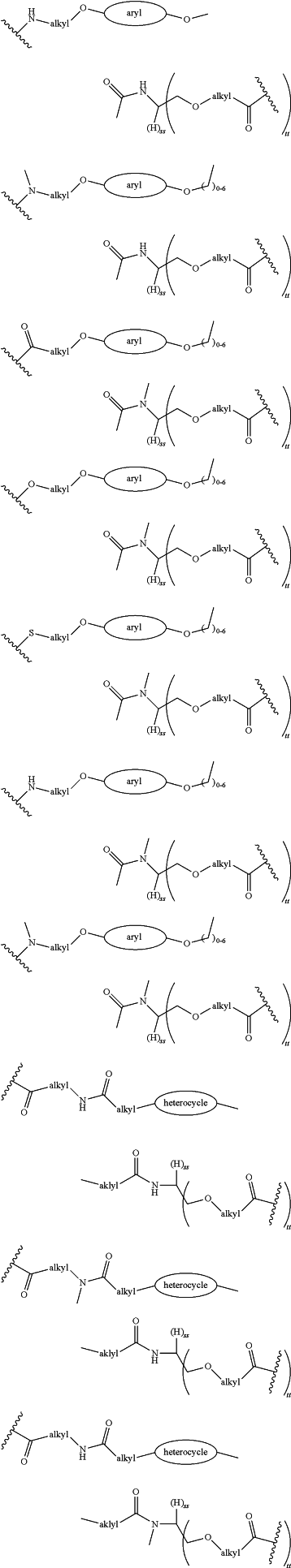   and
the optional substituents are selected from alkyl, alkenyl, alkynyl, haloalkyl, —OR6, F, Cl, Br, I, —NR6R7, heteroalkyl, cyano, nitro, C(═O)R3,
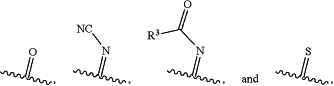 as allowed by valence such that a stable compound results.
|