| CPC A61K 47/549 (2017.08) [A61K 47/545 (2017.08)] | 9 Claims |
|
1. A compound selected from the group consisting of:
     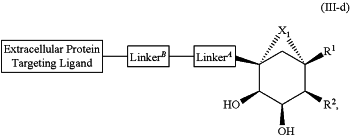        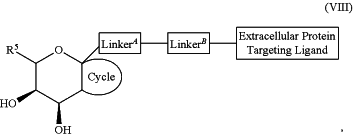                 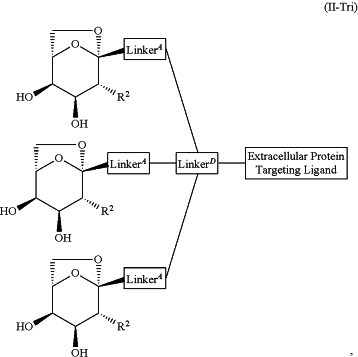   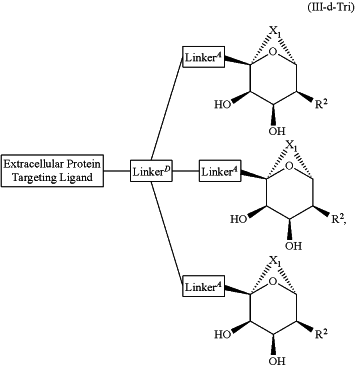         and a pharmaceutically acceptable salt thereof,
wherein:
Extracellular Protein Targeting Ligand is an anti-β1AR antibody binding moiety LB with the structure
 wherein:
AA is an amino acid sequence at least 80% homologous to SEQ ID NO: 1; and
m is 2;
X1 is 1 to 5 contiguous groups independently selected from the group consisting of O, S, N(R6), and C(R4)(R4), wherein:
if X1 is 1 group then X1 is O, S, N(R6), or C(R4)(R4),
if X1 is 2 contiguous groups, then no more than 1 group of X1 is O, S, or N(R6)
a if X1 is 3, 4, or 5 contiguous groups, then no more than 2 groups of X1 are O, S, or N(R6);
R2 is independently selected from the group consisting of:
(i) aryl, heterocycle, and heteroaryl containing 1 or 2 heteroatoms independently selected from the group consisting of N, O, and S, each aryl, heterocycle, and heteroaryl is optionally substituted with 1, 2, 3, or 4 substituents;
(ii)
 (iii) —NR8—S(O)—R3, —NR8—C(S)—R3, —NR8—S(O)(NR6)—R3, —N═S(O)(R3)2, —NR8C(O)NR9S(O)2R3, —NR8—S(O)2—R10, and —NR8—C(NR6)—R3,
and each is optionally substituted with 1, 2, 3, or 4 substituents; and
(iv) hydrogen, R10, alkyl-C(O)—R3, —C(O)—R3, alkyl, haloalkyl, —OC(O)R3, and —NR8—C(O)R10;
R10 is independently selected from the group consisting of alkenyl, allyl, alkynyl, —NR6-alkenyl, —O-alkenyl, —NR6-alkynyl, —NR6-heteroaryl, —NR6-aryl, —O-heteroaryl, —O-aryl, and —O-alkynyl,
each R10 is optionally substituted with 1, 2, 3, or 4 substituents;
R1 and R5 are each independently selected from the group consisting of hydrogen, heteroalkyl, C0-C6alkyl-cyano, alkyl, alkenyl, alkynyl, haloalkyl, F, Cl, Br, I, aryl, arylalkyl, heteroaryl, heteroarylalkyl, heterocycle, heterocycloalkyl, haloalkoxy, —O-alkenyl, —O-alkynyl, C0-C6alkyl-OR6, C0-C6alkyl-SR6, C0-C6alkyl-NR6R7, C0-C6alkyl-C(O)R3, C0-C6alkyl-S(O)R3, C0-C6alkyl-C(S)R3, C0-C6alkyl-S(O)2R3, C0-C6alkyl-N(R8)—C(O)R3, C0-C6alkyl-N(R8)—S(O)R3, C0-C6alkyl-N(R8)—C(S)R3, C0-C6alkyl-N(R8)—S(O)2R3 C0-C6alkyl-O—C(O)R3, C0-C6alkyl-O—S(O)R3, C0-C6alkyl-O—C(S)R3, —N═S(O)(R3)2, C0-C6alkylN3, and C0-C6alkyl-O—S(O)2R3,
and each is optionally substituted with 1, 2, 3, or 4 substituents;
each occurrence of R3 is independently selected from the group consisting of hydrogen, alkyl, heteroalkyl, haloalkyl, arylalkyl, heteroarylalkyl, alkenyl, alkynyl, aryl, heteroaryl, heterocycle, —OR8, and —NR8R9;
each occurrence of R4 is independently selected from the group consisting of hydrogen, heteroalkyl, alkyl, haloalkyl, arylalkyl, heteroarylalkyl, alkenyl, alkynyl, aryl, heteroaryl, heterocycle, —OR6, —NR6R7, C(O)R3, S(O)R3, C(S)R3, and S(O)2R3;
each occurrence of R6 and R7 is independently selected from the group consisting of hydrogen, heteroalkyl, alkyl, arylalkyl, heteroarylalkyl, alkenyl, alkynyl, aryl, haloalkyl, heteroaryl, heterocycle, -alkyl-OR8, -alkyl-NR8R9, C(O)R3, S(O)R3, C(S)R3, and S(O)2R3;
each occurrence of R8 and R9 is independently selected from the group consisting of hydrogen, heteroalkyl, alkyl, arylalkyl, heteroarylalkyl, alkenyl, alkynyl, aryl, heteroaryl, and heterocycle;
Cycle is a 3-8 membered fused cyclic group optionally substituted with 1, 2, 3, or 4 substituents;
LinkerA is a bond or is selected from the group consisting of:
   LinkerB is a bond or is selected from the group consisting of:
 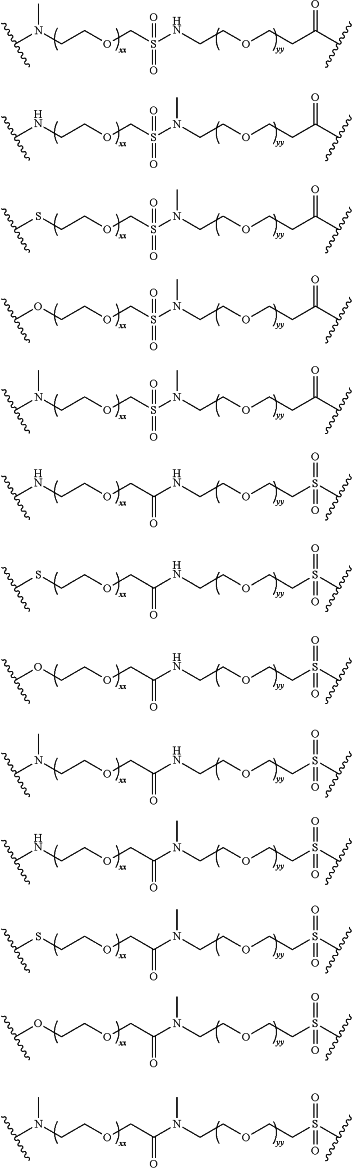  LinkerB is selected from the group consisting of:
     LinkerD is selected from the group consisting of
   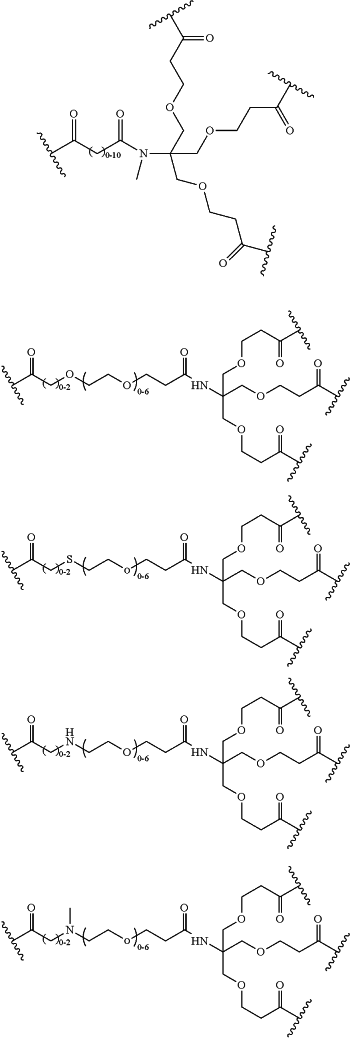 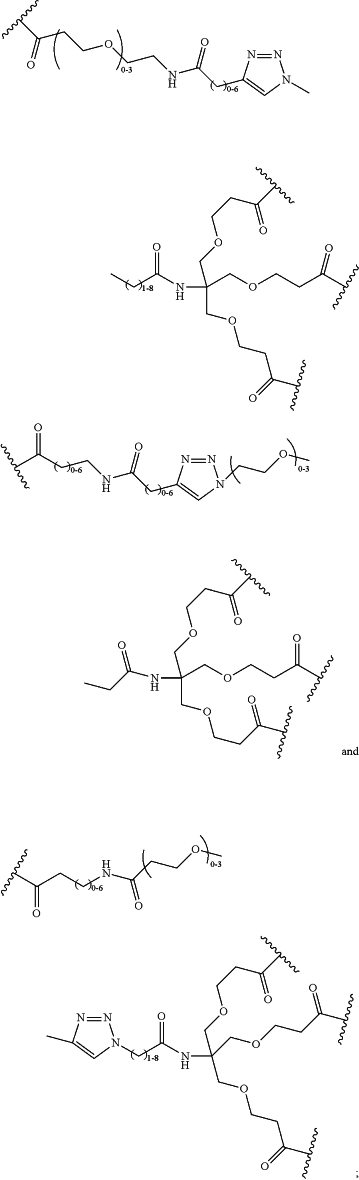 xx is independently at each occurrence 0, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, or 25;
yy is independently at each occurrence 0, 1, 2, 3,4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, or 25;
wherein the optional substituents are selected from the group consisting of alkyl, alkenyl, alkynyl, haloalkyl, —OR6, F, Cl, Br, I, —NR6R7, heteroalkyl, cyano, nitro, —C(O)R3, —C(═O)—, —C(═N—C(═O)R3)—, and —C(═S), as allowed by valence such that a stable compound results.
|