| CPC A61K 31/5415 (2013.01) [A23L 33/105 (2016.08); A23L 33/12 (2016.08); A23L 33/15 (2016.08); A23P 10/28 (2016.08); A23P 10/30 (2016.08); A61K 45/06 (2013.01); A61P 25/28 (2018.01); B65D 75/36 (2013.01); A23V 2002/00 (2013.01); B65D 2203/02 (2013.01)] | 20 Claims |
|
1. A method of therapeutic treatment of a neurodegenerative disorder of protein aggregation in a subject,
which method comprises orally administering two or more times per day to said subject a methylthioninium (MT)-containing compound,
wherein said administration provides a total daily dose of between 0.5 and 20 mg of MT to the subject per day,
wherein the MT-containing compound is a compound of the following formula (“LMTX”):
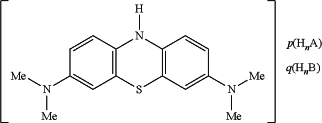 wherein each of HnA and HnB (where present) are protic acids which may be the same or different,
and wherein p=1 or 2; q=0 or 1; n=1 or 2; (p+q)×n=2,
and wherein said neurodegenerative disorder is selected from the group consisting of: Alzheimer's disease, Pick's disease, progressive supranuclear palsy, frontotemporal dementia (FTD), FTD with parkinsonism linked to chromosome 17, frontotemporal lobar degeneration syndromes, disinhibition-dementia-parkinsonism-amyotrophy complex, pallido-ponto-nigral degeneration, Guam-ALS syndrome, pallido-nigro-luysian degeneration, cortico-basal degeneration, dementia with argyrophilic grains, dementia pugilistica, chronic traumatic encephalopathy, Down's syndrome, subacute sclerosing panencephalitis, Niemann-Pick disease type C, Sanfilippo syndrome type B, a myotonic dystrophy DM1 or DM2, Huntington's disease, spinal bulbar muscular atrophy, dentatorubropallidoluysian atrophy, spinocerebellar ataxias, a TDP-43 proteinopathy which is FTLD-TDP, Parkinson's disease, dementia with Lewy bodies, multiple system atrophy, hereditary cerebral angiopathy, amyotrophic lateral sclerosis, and familial encephalopathy with neuronal inclusion bodies.
|