| CPC A61K 31/4164 (2013.01) [A61K 31/4178 (2013.01); A61K 31/439 (2013.01); A61K 31/4439 (2013.01); A61K 31/454 (2013.01); A61K 31/46 (2013.01); A61K 31/5377 (2013.01); A61K 31/55 (2013.01); C07D 233/28 (2013.01); C07D 235/02 (2013.01); C07D 401/06 (2013.01); C07D 401/12 (2013.01); C07D 403/06 (2013.01); C07D 403/12 (2013.01); C07D 405/12 (2013.01); C07D 409/12 (2013.01); C07D 471/08 (2013.01); C07D 491/107 (2013.01)] | 23 Claims |
|
1. A compound of formula (I):
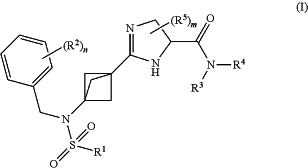 or a pharmaceutically acceptable salt or a solvate thereof, wherein:
R1 represents C1-6 alkyl, C1-6 alkoxy, C1-6 alkanol, —X—C3-8 cycloalkyl, haloC1-6 alkyl, aryl, heterocyclyl or heteroaryl, wherein said cycloalkyl, aryl, heterocyclyl or heteroaryl groups may be optionally substituted by one or more Ra groups;
Ra represents C1-6 alkyl, halogen, haloC1-6 alkyl, hydroxy, cyano, nitro, oxo, CONRxRy or C3-8 cycloalkyl;
Rx and Ry independently represent hydrogen or C1-6 alkyl;
X represents a bond, —CH2— or —(CH2)2—;
R2 represents halogen, haloC1-6 alkyl or cyano;
n represents an integer selected from 0 to 4;
R3 represents hydrogen, C1-6 alkyl, —X—C3-8 cycloalkyl, haloC1-6 alkyl or —X-aryl,
wherein said alkyl may be optionally substituted by one or more cycloalkyl groups,
wherein said cycloalkyl may be optionally substituted by one or more C1-6 alkyl, C1-6 alkoxy, haloC1-6 alkyl, halogen, hydroxy or cyano groups,
wherein said haloalkyl may be optionally substituted by one or more hydroxy groups,
wherein said aryl may be optionally substituted by one or more halogen groups,
wherein R3 and R4 together with the nitrogen atom to which they are attached may join to form a heterocyclyl ring optionally substituted by one or more C1-6 alkyl, haloC1-6 alkyl, or halogen;
R4 represents hydrogen, C1-6 alkyl or C3-8 cycloalkyl;
R5 represents C1-6 alkyl or —X-aryl; and
m represents an integer selected from 0 to 4, such that when m represents 2, said R5 groups may join to form a C3-8 cycloalkyl group.
|