| CPC A61K 31/4045 (2013.01) [A61K 9/006 (2013.01); A61K 9/7007 (2013.01); A61K 47/10 (2013.01); A61K 47/12 (2013.01); A61K 47/26 (2013.01); A61K 47/32 (2013.01); A61K 47/38 (2013.01); A61K 47/42 (2013.01)] | 20 Claims |
|
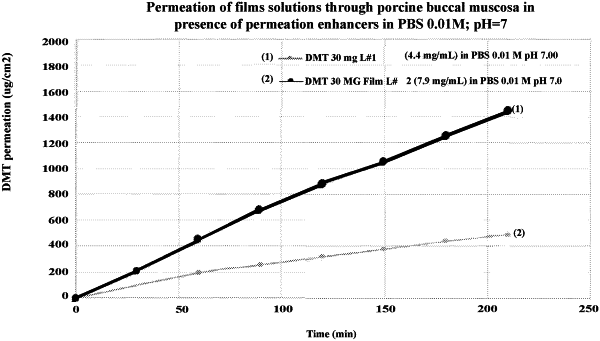
1. A mucoadhesive therapeutic composition comprising:
a pharmaceutically effective amount of an active DMT agent, the active DMT agent comprising N, N-dimethyltryptamine or a pharmaceutically acceptable salt thereof, wherein the pharmaceutically effective amount of the active DMT agent is about 0.5% to about 60% by weight of the mucoadhesive therapeutic composition; and
a polymeric carrier matrix,- the polymeric carrier matrix stabilizing the DMT agent in an amorphous form within the mucoadhesive therapeutic composition, wherein the polymeric carrier matrix is present in the mucoadhesive therapeutic composition in an amount of 15 to 80 percent by weight of the mucoadhesive therapeutic composition, the polymeric carrier matrix comprising at least one cellulose derivative and a copolymer of N-vinyl-2-pyrrolidone and vinyl acetate, the cellulose derivative being selected from hydroxyethyl cellulose, hydroxypropyl cellulose, hydroxypropyl methylcellulose, or combinations thereof,
wherein the stabilization of the DMT agent in an amorphous form is characterized by a powder x-ray diffractogram free of any discernable peaks that are attributable to the active DMT agent.
|