| CPC H01L 23/3735 (2013.01) [B32B 15/00 (2013.01); B32B 15/04 (2013.01); B32B 15/043 (2013.01); B32B 15/18 (2013.01); B32B 18/00 (2013.01); C04B 35/645 (2013.01); C04B 37/021 (2013.01); C04B 37/026 (2013.01); H01L 21/4807 (2013.01); H01L 23/12 (2013.01); H01L 23/13 (2013.01); C04B 2235/6567 (2013.01); C04B 2235/6581 (2013.01); C04B 2235/72 (2013.01); C04B 2235/725 (2013.01); C04B 2237/12 (2013.01); C04B 2237/123 (2013.01); C04B 2237/126 (2013.01); C04B 2237/127 (2013.01); C04B 2237/343 (2013.01); C04B 2237/366 (2013.01); C04B 2237/368 (2013.01); C04B 2237/407 (2013.01); C04B 2237/50 (2013.01); C04B 2237/52 (2013.01); C04B 2237/525 (2013.01); C04B 2237/60 (2013.01); C04B 2237/704 (2013.01); H01L 23/15 (2013.01); H01L 23/36 (2013.01); H01L 2224/32225 (2013.01); H05K 1/0306 (2013.01); H05K 3/38 (2013.01); Y10T 428/12576 (2015.01); Y10T 428/12597 (2015.01); Y10T 428/12611 (2015.01); Y10T 428/12618 (2015.01); Y10T 428/12882 (2015.01); Y10T 428/12903 (2015.01); Y10T 428/1291 (2015.01); Y10T 428/24967 (2015.01); Y10T 428/265 (2015.01)] | 8 Claims |
|
1. A copper/ceramic bonded body, the copper and the ceramic bonded through a bonding material which contains Mg alone or a combination of Mg and an active metal, comprising:
a copper member made of copper or a copper alloy; and
a ceramic member containing Al or Si, the copper member and the ceramic member being bonded to each other,
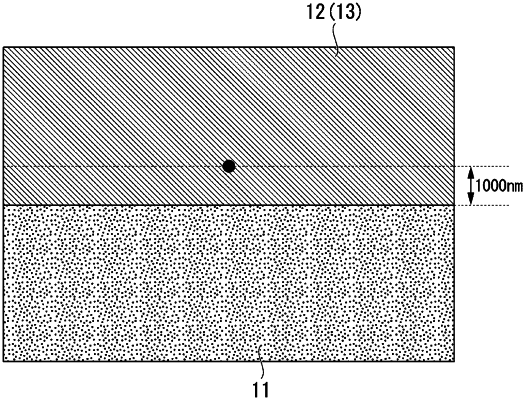
wherein a total concentration of Al, Si, Zn, and Mn is 3 atom % or less when concentration measurement is performed by an energy dispersive X-ray analysis method at a position 1000 nm away from a bonded interface between the copper member and the ceramic member to a copper member side, assuming that a total value of Cu, Mg, Ti, Zr, Nb, Hf, Al, Si, Zn, and Mn is 100 atom %,
the bonding material is distributed in the copper member and the ceramic member,
the active metal is one or more selected from Ti, Zr, Hf, and Nb, and
the ceramic member is made of AlN, Al2O3, or Si3N4.
|