| CPC G03F 7/0382 (2013.01) [C23C 14/04 (2013.01); C23C 14/30 (2013.01); C23C 14/34 (2013.01); G03F 7/0045 (2013.01); G03F 7/0046 (2013.01); G03F 7/168 (2013.01); G03F 7/2004 (2013.01); G03F 7/322 (2013.01); G03F 7/38 (2013.01)] | 30 Claims |
|
1. A chemically-amplified, negative-acting, photoresist composition, imageable by 365 nm radiation that is developable in aqueous base, the photoresist composition comprising solids components a), b), c) d) and e) and solvent component f);
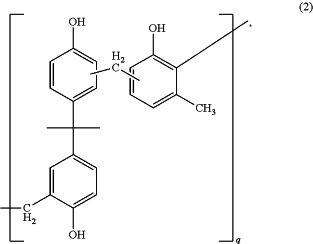
a) an aqueous base soluble phenolic film-forming polymeric binder resin having ring bonded hydroxyl groups which is a base soluble Novolak, wherein said binder resin, comprises repeat units of structure (2), wherein q is the number of repeat units in a polymer chain,
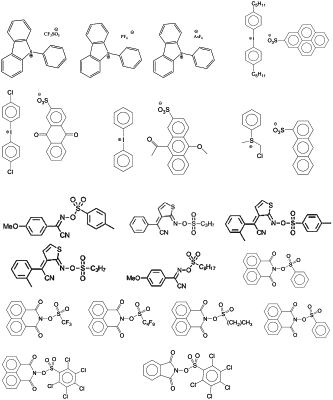
 b) a photoacid generator based on a trihalomethyl derivative, wherein the halo moiety is chlorine or bromine and that forms an acid upon exposure to radiation at 365 nm, in an amount sufficient to initiate crosslinking of the film-forming binder resin;
c) a crosslinking agent that forms a carbonium ion upon exposure to the acid from step b) generated by exposure to radiation, and which comprises an etherified melamine;
d) a dye having a molar attenuation coefficient at 365 nm ranging from about 1.74×104 to about 0.94×104 mole −1 L cm−1 as measured in PGMEA, which is soluble in aqueous base;
e) a quencher system consisting essentially of an amine quencher, or a mixture of such amine quenchers, having a pKa in water from about 6.0 to about 12, where the amine quencher has a boiling point of at least 100° C., at 1 atmosphere pressure, and comprises at least one C2-C25 alkyl substituent, a —(CH2)nOH substituent, or a —(CH2)n—O—(CH2)n′—O—R′ substituent or comprises a mixture of these substituents, where n and n′ are independently an integer ranging from 2 to 4 and R′ is a C1-C4 alkyl or H;
f) a photoresist solvent.
|