| CPC G01N 33/547 (2013.01) [C12N 9/0006 (2013.01); C12N 9/0065 (2013.01); C12N 9/16 (2013.01); C12Q 1/6804 (2013.01); C12Q 1/682 (2013.01); G01N 33/533 (2013.01); G01N 33/535 (2013.01); G01N 33/54353 (2013.01); C12Y 101/03004 (2013.01); C12Y 111/01007 (2013.01); C12Y 301/03001 (2013.01)] | 17 Claims |
|
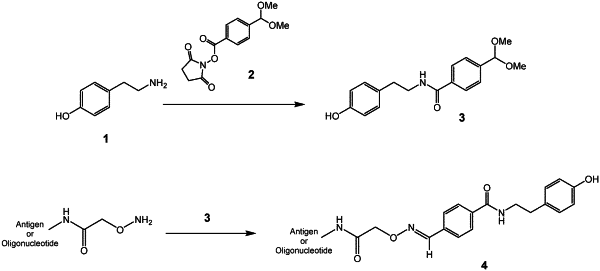
1. A diagnostic kit comprising:
a first reagent compound comprising a bridging peptide coupled to a tyramide moiety, said bridging peptide being selected from the group consisting of SEQ ID NO: 1, SEQ ID NO: 2, SEQ ID NO: 3, SEQ ID NO: 4 and SEQ ID NO: 5;
a first detectable antibody having specificity for the bridging peptide with high affinity, wherein the first detectable antibody comprises a detectable label, said first detectable antibody being a monoclonal antibody; and
instructions for use.
|