| CPC C07D 487/04 (2013.01) [C07B 2200/13 (2013.01)] | 31 Claims |
|
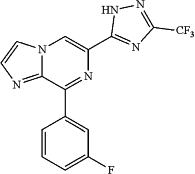
1. A crystalline Hydrate 2 of 8-(2-fluorobenzyl)-6-(3-(trifluoromethyl)-1H-1,2,4-triazol-5-yl)imidazo[1,2-a]pyrazine of the formula:
 (i) wherein the crystalline Hydrate 2 is characterized by a powder X-ray diffraction pattern comprising at least three diffraction peaks at diffraction angles (°2θ) selected from the group consisting of 10.4°±0.2 °2θ, 15.50±0.2 °2θ, 18.4°±0.2 °2θ, 18.7°±0.2 °2θ, and 21.9°±0.2 °2θ; or
(ii) wherein the crystalline Hydrate 2 is characterized by a powder X-ray diffraction pattern comprising at least three diffraction peaks at diffraction angles (°2θ) selected from the group consisting of 7.3°±0.2 °2θ, 10.6°±0.2 °2θ, 15.8°±0.2 °2θ, 15.9°±0.2 °2θ, and 27.6°±0.2 °2θ.
|
|
30. A pharmaceutical composition comprising the crystalline Hydrate 2 of claim 1 and a pharmaceutically acceptable carrier or diluent.
|