| CPC C07D 211/62 (2013.01) [C07D 401/04 (2013.01); C07D 401/06 (2013.01); C07D 405/12 (2013.01); C07D 405/14 (2013.01); C07D 471/04 (2013.01)] | 28 Claims |
|
1. A compound of formula (I):
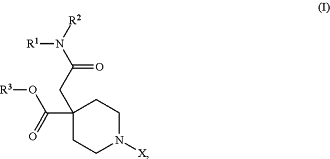 wherein:
R1 is C1-C6 alkyl, C3-C6 cycloalkyl, C3-C9 heterocycloalkyl, or C6-C10 aryl, wherein the C1-C6 alkyl, C3-C6 cycloalkyl, C3-C9 heterocycloalkyl, or C6-C10 aryl is optionally substituted with one or more R1a;
each R1a independently is halogen, C1-C6 alkyl, C1-C6 alkoxyl, or C3-C6 cycloalkyl;
R2 is C1-C6 alkyl, C3-C6 cycloalkyl, C3-C9 heterocycloalkyl, or C6-C10 aryl, wherein the C1-C6 alkyl, C3-C6 cycloalkyl, C3-C9 heterocycloalkyl, or C6-C10 aryl is optionally substituted with one or more R2a;
each R2a independently is halogen, C1-C6 alkyl, C1-C6 alkoxyl, or C3-C6 cycloalkyl;
R3 is H or C1-C6 alkyl;
X is C5-C10 heteroaryl, C6-C10 aryl,
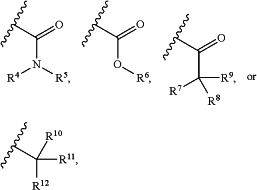 wherein the C5-C10 heteroaryl or C6-C10 aryl is optionally substituted with one or more Xa;
each Xa independently is halogen, C1-C6 alkyl, C1-C6 alkoxyl, or C3-C6 cycloalkyl;
R4 is H, C1-C6 alkyl, C3-C6 cycloalkyl, or C6-C10 aryl, wherein the C1-C6 alkyl, C3-C6 cycloalkyl, or C6-C10 aryl is optionally substituted with one or more R4a;
each R4a independently is halogen, cyano, C1-C6 alkyl, C1-C6 alkoxyl, or C3-C6 cycloalkyl;
R5 is H, C1-C6 alkyl, C3-C6 cycloalkyl, or C6-C10 aryl, wherein the C1-C6 alkyl, C3-C6 cycloalkyl, or C6-C10 aryl is optionally substituted with one or more R5a;
each R5a independently is halogen, cyano, C1-C6 alkyl, C1-C6 alkoxyl, or C3-C6 cycloalkyl;
R6 is H or C1-C6 alkyl;
R7 is C1-C6 alkyl, C3-C6 cycloalkyl, or C6-C10 aryl, wherein the C1-C6 alkyl, C3-C6 cycloalkyl, or C6-C10 aryl is optionally substituted with one or more R7a;
each R7a independently is halogen, cyano, or C3-C6 cycloalkyl;
R8 is H, halogen, or C1-C6 alkyl;
R9 is H, halogen, or C1-C6 alkyl, wherein R8 and R9 may alternatively come together to form a C3-C6 cycloalkyl;
R10 and R11 are each H or R10 and R11 together form an oxo;
R12 is C1-C6 alkyl, C3-C6 cycloalkyl, C6-C10 aryl, C1-C3-(phenyl), or C3-C10 heterocycloalkyl wherein the C1-C6 alkyl, C3-C6 cycloalkyl, C6-C10 aryl, C1-C3-(phenyl), or C3-C10 heterocycloalkyl is optionally substituted with one or more R12a; and
each R12a independently is halogen, cyano, C1-C6 alkyl, C1-C6 alkoxyl, or C3-C6 cycloalkyl,
or a pharmaceutically acceptable salt thereof.
|