| CPC A61M 39/20 (2013.01) [A61M 39/16 (2013.01); A61M 39/162 (2013.01); A61M 39/165 (2013.01)] | 8 Claims |
|
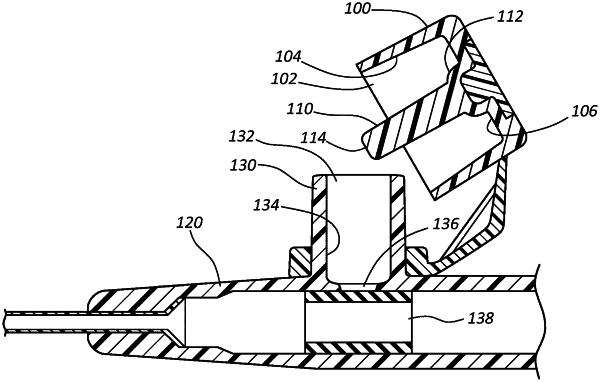
1. An antimicrobial catheter system, comprising:
a catheter adapter, comprising:
an interior lumen;
a side port; and
a port valve forming a defeatable seal between the side port and the interior lumen;
an antimicrobial cap coupled to the side port, the antimicrobial cap comprising:
an opening having a diameter that receives the side port;
an inner surface extending from one side of the opening to another side of the opening opposite the one side, the inner surface defining a volume, wherein the side port is received into the volume; and
a quantity of antimicrobial material applied to the inner surface, wherein the quantity of antimicrobial material is configured to be dissolved into a residual fluid from the side port to provide an antimicrobial solution within the volume having a concentration from approximately 0.005% w/w to approximately 25% w/w.
|