| CPC A61K 31/4184 (2013.01) [A61K 31/4192 (2013.01); A61K 31/4196 (2013.01); A61K 31/422 (2013.01); A61K 31/424 (2013.01); A61K 31/437 (2013.01); A61K 31/4439 (2013.01); A61K 31/444 (2013.01); A61K 45/06 (2013.01); C07D 405/10 (2013.01); C07D 405/14 (2013.01); C07D 409/14 (2013.01); C07D 413/10 (2013.01); C07D 471/04 (2013.01); C07D 493/04 (2013.01); C07D 493/10 (2013.01); C07D 498/04 (2013.01)] | 39 Claims |
|
1. A compound of Formula (I):
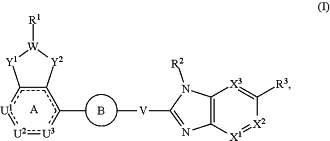 or a pharmaceutically acceptable salt thereof, wherein
R1 is C1-6 alkyl, C1-6 haloalkyl, C2-6 alkenyl, C2-6 alkynyl, C3-10 cycloalkyl, heterocyclyl, C6-10 aryl, heteroaryl, —C(O)N(R1b)(R1c), —C(O)R1b, or —C(O)OR1c,
wherein the alkyl, haloalkyl, cycloalkyl, aryl, heteroaryl, or heterocyclyl is each optionally substituted with one to four Z1;
ring A is an aromatic ring in which U1, U2, U3, are each independently —C(H)═, —C(Z1a)═, or —N═;
ring B is C6-10 aryl or heteroaryl, which is each optionally substituted with one to four R4;
R2 is H, C1-6 alkyl, C1-6 haloalkyl, C2-6 alkenyl, C2-6 alkynyl, C3-10 cycloalkyl, heterocyclyl, C6-10 aryl, heteroaryl, —S—R2a, —S(O)R2a, —S(O)(NH)R2a, —S(O)2R2a, —S(O)2N(R2a)(R2b), or —S(O)(NR2a)R2b,
wherein the alkyl, cycloalkyl, heterocyclyl, aryl, or heteroaryl is each optionally substituted with one to four Z1;
X1, X2, and X3 are each independently —N═, —C(H)═, or —C(R8)═;
Y1 and Y2 are each —C(Ry1)(Ry2)—, —N(Ry1)—, —O—, —S—, —S(O)2—, or —C(O)—;
W is —C(R5)—, or —N—,
wherein when W is —N, one of Y1 and Y2 is —C(Ry1) (Ry2)— or —C(O)— and the other of Y1 and Y2 is —C(Ry1) (Ry2)—, —C(O)—, or —S(O)2—;
R3 is H, C1-6 alkyl, C1-6 haloalkyl, C2-6 alkenyl, C2-6 alkynyl, halogen, C3-10 cycloalkyl, heterocyclyl, C6-10 aryl, heteroaryl, —CN, —NO2, —OR3a, —C(O)R3a, —C(O)OR3a, —C(O)N(R3a) (R3b), —N(R3a)C(O)R3b, —N(R3a)C(O)OR3b, —N(R3a)C(O)N(R3b)2, —C(O)NHS(O)2R3a, —C(O)NR3aS(O)2R3b, —C(O)NR3aS(O)2NR3bR3c, —S(O)2OR3a, —S(O)2N(R3a)(R3b), —N(R3a)S(O)2R3b, —S(O)2NHC(O)R3a, —S(O)(═NR3a)R3b, —S(═NR3a)(═NR3b)R3c, —P(O)(OR3a)(R3b), —P(O)(OR3a)(OR3b), or —B(OR3a)(OR3b), wherein the alkyl, haloalkyl, alkenyl, alkynyl, cycloalkyl, heterocyclyl, aryl, or heteroaryl is each optionally substituted with one to four R3d;
each R3a, R3b, and R3c is independently H, C1-6 alkyl, C1-6 haloalkyl, C2-8 alkoxyalkyl, —C1-4 alkyl-N(R9a)(R9b), —C1-4 alkyl-C(O)N(R9a) (R9b), —C1-4 alkyl-O—C(O)—C1-4 alkyl, —C1-4 alkyl-O—C(O)—O—C1-4alkyl, —C1-4 alkyl-O—C(O)—C1-4 alkyl-N(R9a) (R9b), —C1-4 alkyl-C3-8 cycloalkyl, —C1-4 alkyl-heterocyclyl, C2-6 alkenyl, C2-6 alkynyl, C3-10 cycloalkyl, heterocyclyl, C6-10 aryl, heteroaryl, —P(O)(OR9c)2, —OP(O)(OR9c)2, —CH2P(O)(OR9c)2, —OCH2P(O)(OR9c)2, —C(O)OCH2P(O)(OR9c)2, —P(O)(R9c)(OR9d), —OP(O)(R9c)(OR9d), —CH2P(O)(R9c)(OR9d), —OCH2P(O)(R9c)(OR9d), —C(O)OCH2P(O)(R9c)(OR9d), —P(O)(N(R9c)2)2, —OP(O)(N(R9c)2)2, —CH2P(O)(N(R9c)2)2, —OCH2P(O)(N(R9c)2)2, —C(O)OCH2P(O)(N(R9c)2)2, —P(O)(N(R9c)2)(OR9d), —OP(O)(N(R9c)2)(OR9d), —CH2P(O)(N(R9c)2)(OR9d), —OCH2P(O)(N(R9c)2)(OR9d), —C(O)OCH2P(O)(N(R9c)2)(OR9d), —P(O)(R9c)(N(R9d)2), —OP(O)(R9c)(N(R9d)2), —CH2P(O)(R9c)(N(R9d)2), —OCH2P(O)(R9c)(N(R9d)2), or —C(O)OCH2P(O)(R9c)(N(R9d)2);
wherein the alkyl, alkenyl, cycloalkyl, heterocyclyl, aryl, or heteroaryl is each optionally substituted with one to four Z1b,
each R4 is independently C1-9 alkyl, C1-8 haloalkyl, C1-6 haloalkoxy, C2-6 alkoxyalkyl, C2-6 alkenyl, C2-6 alkynyl, halogen, C3-15 cycloalkyl, heterocyclyl, C6-10 aryl, heteroaryl, oxo, —NO2, —CN, —N3, —O—R4a, —C(O)R4a, —C(O)O—R4a, —C(O)N(R4a) (R4b), —N(R4a) (R4b), —N(R4a)2(R4b)+, —N(R4a)—C(O)R4b, —N(R4a)C(O)O(R4b), —N(R4a)C(O)N(R4b)(R4c), —N(R4a)S(O)2(R4b), —N(R4a)S(O)2—N(R4b)(R4c), —N(R4a)S(O)2O(R4b), —OC(O)R4a, —OC(O)OR4a, —OC(O)—N(R4a) (R4b), —S—R4a, —S(O)R4a, —S(O)(NH)R4a, —S(O)2R4a, —S(O)2N(R4a)(R4b), —S(O)(NR4a)R4b, or —Si(R4a)3;
wherein the alkyl, haloalkyl, alkenyl, alkynyl, cycloalkyl, heterocyclyl, aryl, or heteroaryl is each optionally substituted with one to four Z1b;
or two R4 groups attached to adjacent ring atoms are combined with the atoms to which they are attached to form a C5-10 cycloalkyl or heterocyclyl, which is each optionally substituted with one to four Z1b;
R5 is H, cyclopropyl, or C1-3alkyl, wherein the C1-3alkyl is optionally substituted with one, two or three groups selected from halogen, —OH, —OCH3, —CN, oxo, and —N(Rx1)(Rx2);
or R5 and Ry1 are combined with the atoms to which they are attached to form a C3-10 cycloalkyl or heterocyclyl optionally substituted with oxo;
Rx1 and Rx2 are each independently H, C1-6 alkyl, C3-10 cycloalkyl, heterocyclyl, —S(O)2R6a1, or —S(O)2N(R6a1)(NR6a2), wherein the C1-6 alkyl, cycloalkyl or heterocyclyl is each optionally substituted with F, —CN, oxo, or C3-6 cycloalkyl;
or Rx1 and Rx2 are combined with the atom to which they are attached to form a heterocyclyl, which is optionally substituted with one to four R6b1;
V is —C(O)—, —O—, —N(R6a)—, or —C(R6b) (R6c)—;
R6a is H, C1-6 alkyl, C3-10 cycloalkyl, heterocyclyl, —S(O)2R6a1, or —S(O)2N(R6a1)(NR6a2), wherein the cycloalkyl or heterocyclyl is each optionally substituted with C1-6 alkyl, F, or —CN;
each R6b and R6c is independently H, C1-6 alkyl, C1-6 haloalkyl, C2-6 alkoxyalkyl, halogen, C3-10 cycloalkyl, heterocyclyl, —C1-6 alkyl-N(R9a) (R9b), —CN, —OR6c1, or —N(R6c2) (R6c3), wherein the alkyl, cycloalkyl, or heterocyclyl is each optionally substituted with one to four R6b1;
or R6b and R6c are combined with the atom to which they are attached to form C3-10 cycloalkyl or heterocyclyl, which is each optionally substituted with one to four R6b1;
or R6a or R6c is combined with one R4 group and the atoms to which they are attached to form a C5-10 cycloalkyl or heterocyclyl, which is each optionally substituted with one to four R10;
each Ry1 and Ry2 is independently H, halo, C1-6 alkyl, C1-6 haloalkyl, wherein the alkyl and haloalkyl are each optionally substituted with oxo;
each R3d, R6b1, and R10 is independently C1-6 alkyl, C1-6 haloalkyl, C1-6 alkoxy, C1-6 haloalkoxy, C2-6 alkoxyalkyl, C2-6 alkenyl, C2-6 alkynyl, halogen, C3-10 cycloalkyl, heterocyclyl, C6-10 aryl, heteroaryl, oxo, —OH, —CN, —NO2, or —C(O)N(R2a)(R2b), wherein the heterocyclyl or heteroaryl is optionally substituted with C1-6 alkyl, C1-6 haloalkyl, or C1-6 haloalkoxy; and
each R6a1, R6a2, R6c1, R6c2, and R6c3 is independently H, C1-6 alkyl or C3-10 cycloalkyl;
each R9a and R9b is independently H, C1-6 alkyl, or C1-6 haloalkyl;
each Z1 is independently C1-9 alkyl, C1-8 haloalkyl, C1-6 alkoxy, C1-6 haloalkoxy, C2-6 alkoxyalkyl, C2-6 alkenyl, C2-6 alkynyl, halogen, C3-15 cycloalkyl, heterocyclyl, C6-10 aryl, heteroaryl, oxo, —NO2, —N3, —CN, —O—R12a, —C(O)—R12a, —C(O)O—R12a, —C(O)—N(R12a)(R12b), —N(R12a)(R12b), —N(R12a)2(R12b)+, —N(R12a)C(O)—R12b, —N(R12a)C(O)O—R12b, —N(R12a)C(O)N(R12b)(R12c), —N(R12a)S(O)2(R12b), —NR12aS(O)2N(R12b)(R12c), —NR12aS(O)2O(R12b), —OC(O)R12a, —OC(O)OR12a, —OC(O)—N(R12a)(R12b), —S—R12a, —S(O)R12a, —S(O)(NH)R12a, —S(O)2R12a, —S(O)2N(R12a)(R12b), —S(O)(NR12a)R12b, or —Si(R12a)3;
wherein the alkyl, haloalkyl, alkenyl, alkynyl, cycloalkyl, heterocyclyl, aryl, or heteroaryl is each optionally substituted with one to four Z1a;
each Z1a is independently C1-9 alkyl, C1-8 haloalkyl, C1-6 alkoxy, C1-6 haloalkoxy, C2-6 alkoxyalkyl, C2-6 alkenyl, C2-6 alkynyl, halogen, C3-15 cycloalkyl, heterocyclyl, C6-10 aryl, heteroaryl, oxo, —NO2, —CN, —N3, —O—R12a, —C(O)R12a, —C(O)O—R12a, —C(O)N(R12a)(R12b), —N(R12a)(R12b), —N(R12a)2(R12b)+, —N(R12a)—C(O)R12b, —N(R12a)C(O)O(R12b), —N(R12a)C(O)N(R12b)(R12c), —N(R12a)S(O)2(R12b), —N(R12a)S(O)2—N(R12b)(R12c), —N(R12a)S(O)2O(R12b), —OC(O)R12a, —OC(O)OR12a, —OC(O)—N(R12a)(R12b), —S—R12a, —S(O)R12a, —S(O)(NH)R12a, —S(O)2R12a, —S(O)2N(R12a)(R12b), —S(O)(NR12a)R12b, or —Si (R12a)3;
wherein the alkyl, haloalkyl, alkenyl, alkynyl, cycloalkyl, heterocyclyl, aryl, or heteroaryl is each optionally substituted with one to four Z1b;
each R8 or Z1b is independently C1-9 alkyl, C1-8 haloalkyl, C2-6 alkenyl, C2-6 alkynyl, halogen, C3-15 cycloalkyl, heterocyclyl, C6-10 aryl, heteroaryl, oxo, —OH, —CN, —NO2, —NH2, —N3, —SH, —O(C1-9 alkyl), —O(C1-8 haloalkyl), —O(C2-6 alkenyl), —O(C2-6 alkynyl), —O(C3-15 cycloalkyl), —O(heterocyclyl), —O(C6-10 aryl), —O(heteroaryl), —NH(C1-9 alkyl), —NH(C1-8 haloalkyl), —NH(C2-6 alkenyl), —NH(C2-6 alkynyl), —NH(C3-15 cycloalkyl), —NH(heterocyclyl), —NH(C6-10 aryl), —NH(heteroaryl), —N(C1-9 alkyl)2, —N(C1-8 haloalkyl)2, —N(C2-6 alkenyl)2, —N(C2-6 alkynyl)2, —N(C3-15 cycloalkyl)2, —N(heterocyclyl)2, —N(C6-10 aryl)2, —N(heteroaryl)2, —N(C1-9 alkyl)(C1-8 haloalkyl), —N(C1-9 alkyl)(C2-6 alkenyl), —N(C1-9 alkyl)(C2-6 alkynyl), —N(C1-9 alkyl)(C3-15 cycloalkyl), —N(C1-9 alkyl)(heterocyclyl), —N(C1-9 alkyl)(C6-10 aryl), —N(C1-9 alkyl)(heteroaryl), —C(O)(C1-9 alkyl), —C(O)(C1-8 haloalkyl), —C(O)(C2-6 alkenyl), —C(O)(C2-6 alkynyl), —C(O)(C3-15 cycloalkyl), —C(O)(heterocyclyl), —C(O)(C6-10 aryl), —C(O)(heteroaryl), —C(O)O(C1-9 alkyl), —C(O)O(C1-8 haloalkyl), —C(O)O(C2-6 alkenyl), —C(O)O(C2-6 alkynyl), —C(O)O(C3-15 cycloalkyl), —C(O)O(heterocyclyl), —C(O)O(C6-10 aryl), —C(O)O(heteroaryl), —C(O)NH2, —C(O)NH(C1-9 alkyl), —C(O)NH(C1-8 haloalkyl), —C(O)NH(C2-6 alkenyl), —C(O)NH(C2-6 alkynyl), —C(O)NH(C3-15 cycloalkyl), —C(O)NH(heterocyclyl), —C(O)NH(C6-10 aryl), —C(O)NH(heteroaryl), —C(O)N(C1-9 alkyl)2, —C(O)N(C1-8 haloalkyl)2, —C(O)N(C2-6 alkenyl)2, —C(O)N(C2-6 alkynyl)2, —C(O)N(C3-15 cycloalkyl)2, —C(O)N(heterocyclyl)2, —C(O)N(C6-10 aryl)2, —C(O)N(heteroaryl)2, —NHC(O)(C1-9 alkyl), —NHC(O)(C1-8 haloalkyl), —NHC(O)(C2-6 alkenyl), —NHC(O)(C2-6 alkynyl), —NHC(O)(C3-15 cycloalkyl), —NHC(O)(heterocyclyl), —NHC(O)(C6-10 aryl), —NHC(O)(heteroaryl), —NHC(O)O(C1-9 alkyl), —NHC(O)O(C1-8 haloalkyl), —NHC(O)O(C2-6 alkenyl), —NHC(O)O(C2-6 alkynyl), —NHC(O)O(C3-15 cycloalkyl), —NHC(O)O(heterocyclyl), —NHC(O)O(C6-10 aryl), —NHC(O)O(heteroaryl), —NHC(O)NH(C1-9 alkyl), —NHC(O)NH(C1-8 haloalkyl), —NHC(O)NH(C2-6 alkenyl), —NHC(O)NH(C2-6 alkynyl), —NHC(O)NH(C3-15 cycloalkyl), —NHC(O)NH(heterocyclyl), —NHC(O)NH(C6-10 aryl), —NHC(O)NH(heteroaryl), —NHS(O)(C1-9 alkyl), —N(C1-9 alkyl)S(O)(C1-9 alkyl), —S(C1-9 alkyl), —S(C1-8 haloalkyl), —S(C2-6 alkenyl), —S(C2-6 alkynyl), —S(C3-15 cycloalkyl), —S(heterocyclyl), —S(C6-10 aryl), —S(heteroaryl), —S(O)N(C1-9 alkyl)2, —S(O)(C1-9 alkyl), —S(O)(C1-8 haloalkyl), —S(O)(C2-6 alkenyl), —S(O)(C2-6 alkynyl), —S(O)(C3-15 cycloalkyl), —S(O)(heterocyclyl), —S(O)(C6-10 aryl), —S(O)(heteroaryl), —S(O)2(C1-9 alkyl), —S(O)2(C1-8 haloalkyl), —S(O)2(C2-6 alkenyl), —S(O)2(C2-6 alkynyl), —S(O)2(C3-15 cycloalkyl), —S(O)2 (heterocyclyl), —S(O)2(C6-10 aryl), —S(O)2 (heteroaryl), —S(O)(NH) (C1-9 alkyl), —S(O)2NH(C1-9 alkyl), or —S(O)2N(C1-9 alkyl)2;
wherein the alkyl, cycloalkyl, heterocyclyl, aryl, or heteroaryl in each instance is optionally substituted with one to three C1-9 alkyl, C1-8 haloalkyl, halogen, —OH, —NH2, —O(C1-9 alkyl), —O(C1-8 haloalkyl), —O(C3-15 cycloalkyl), —O(heterocyclyl), —O(aryl), —O(heteroaryl), —NH(C1-9 alkyl), —NH(C1-8 haloalkyl), —NH(C3-15 cycloalkyl), —NH(heterocyclyl), —NH(aryl), —NH(heteroaryl), —N(C1-9 alkyl)2, —N(C3-15 cycloalkyl)2, —NHC(O)(C1-8 haloalkyl), —NHC(O)(C3-15 cycloalkyl), —NHC(O)(heterocyclyl), —NHC(O)(aryl), —NHC(O)(heteroaryl), —NHC(O)O(C1-9 alkyl), —NHC(O)O(C1-8 haloalkyl), —NHC(O)O(C2-6 alkynyl), —NHC(O)O(C3-15 cycloalkyl), —NHC(O)O(heterocyclyl), —NHC(O)O(aryl), —NHC(O)O(heteroaryl), —NHC(O)NH(C1-9 alkyl), S(O)2(C1-9 alkyl), —S(O)2(C1-8 haloalkyl), —S(O)2(C3-15 cycloalkyl), —S(O)2 (heterocyclyl), —S(O)2 (aryl), —S(O)2 (heteroaryl), —S(O)(NH) (C1-9 alkyl), —S(O)2NH(C1-9 alkyl), or —S(O)2N (C1-9 alkyl)2; and
each R1b, R1c, R2a, R2b, R4a, R4b, R4c, R9c, R9d, R12a, R12b, and R12c is independently H, C1-9 alkyl, C2-6 alkenyl, C2-6 alkynyl, C3-15 cycloalkyl, heterocyclyl, C6-10 aryl, or heteroaryl wherein the alkyl, alkenyl, alkynyl, cycloalkyl, heterocyclyl, aryl, or heteroaryl is each optionally substituted with one to four Z1b;
wherein each heteroaryl has 5 to 12 ring members and has one to four heteroatoms each independently N, O, or S; and
wherein each heterocyclyl has 3 to 12 ring members and has one to four heteroatoms each independently N, O, or S.
|