| CPC A61N 1/3629 (2017.08) [A61B 5/363 (2021.01); A61K 31/7076 (2013.01); A61M 5/16813 (2013.01); A61N 1/0568 (2013.01); A61M 2205/04 (2013.01); A61M 2205/054 (2013.01); A61M 2205/3523 (2013.01); A61M 2205/50 (2013.01); A61M 2210/125 (2013.01); A61M 2230/06 (2013.01)] | 20 Claims |
|
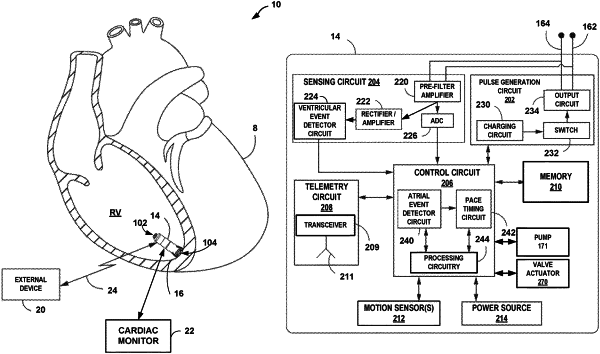
1. An implantable medical device comprising:
processing circuitry configured to detect an occurrence of an arrhythmia in a heart of a patient;
a reservoir containing one or more therapeutically useful doses of a drug for treating the arrhythmia; and
a valve operable to be opened in response to detection of the occurrence of the arrhythmia in the heart of the patient to release a therapeutically useful dose of the drug into the heart of the patient to treat arrhythmia of the heart;
wherein the implantable medical device is adapted for one of implantation wholly within a heart chamber of the heart of the patient or within a pulmonary artery of the patient.
|